Korean Circ J.
2017 Mar;47(2):193-200. 10.4070/kcj.2016.0137.
Inhibiting Cytochrome C Oxidase Leads to Alleviated Ischemia Reperfusion Injury
- Affiliations
-
- 1Department of Anesthesia, Xiang-Ya Second Hospital, Central South University, Changsha, China. lei_liull@sina.com
- 2Department of Anesthesiology, the Second Affiliated Hospital, University of South China, Hengyang, China.
- 3Department of Anesthesiology, Zunyi Medical College, Zunyi, Guizhou, China.
- KMID: 2377459
- DOI: http://doi.org/10.4070/kcj.2016.0137
Abstract
- BACKGROUND AND OBJECTIVES
The overall purpose of this study was to investigate the role of cytochrome C oxidase (CcO) in preventing ischemia reperfusion-induced cardiac injury through gaseous signaling molecule pathways.
MATERIALS AND METHODS
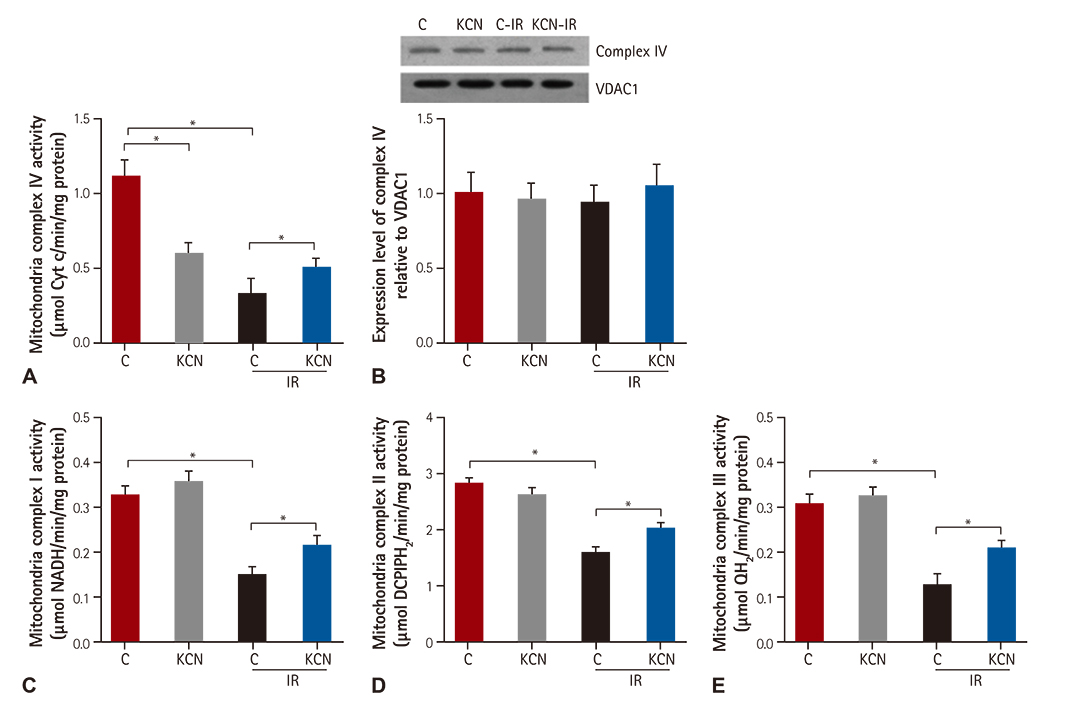
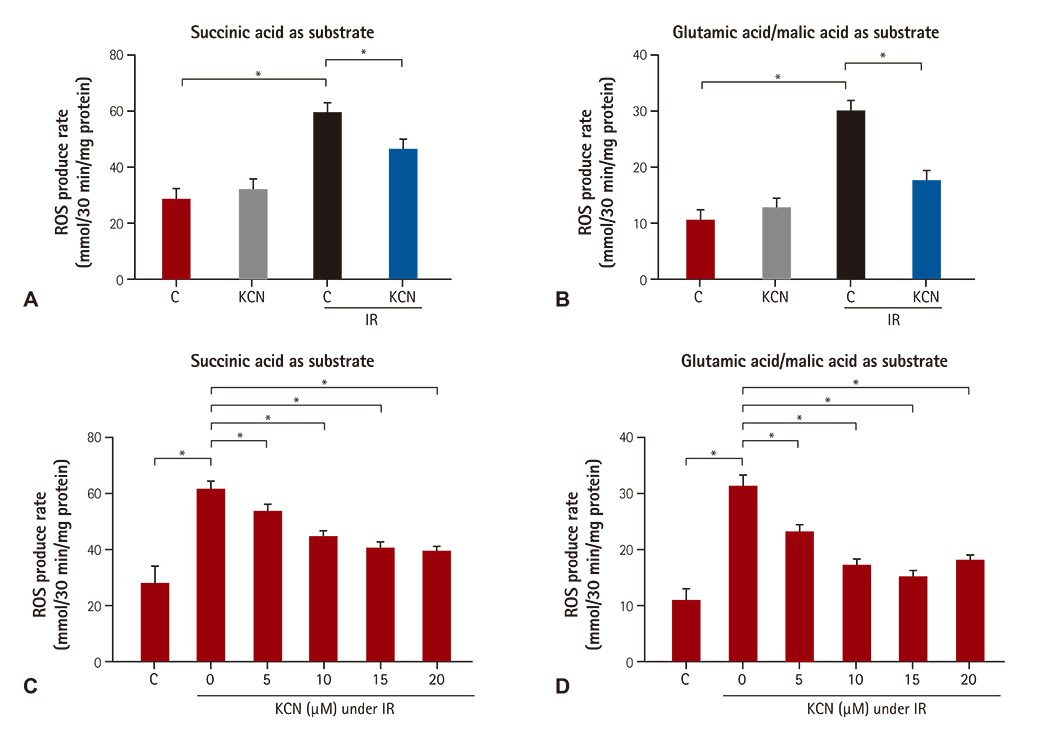
We used CcO inhibitor, potassium cyanide (KCN) to mimic the pre-treatment of gaseous signaling molecules in a global ischemia/reperfusion (IR) injury model in rats. Intracellular reactive oxygen species (ROS) was determined by measuring mitochondrial H2O2 and mitochondrial complex activity.
RESULTS
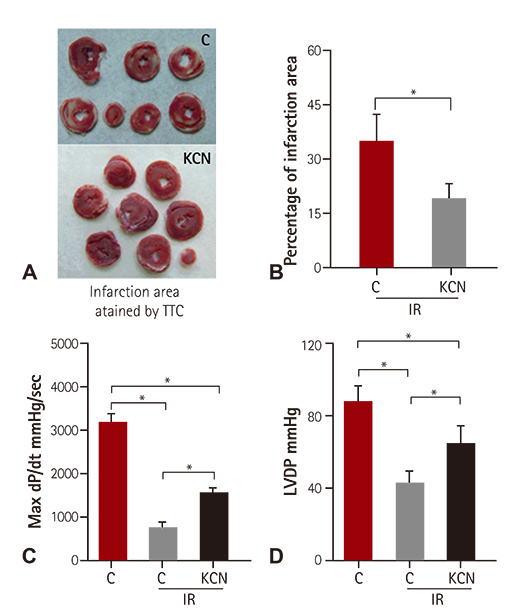
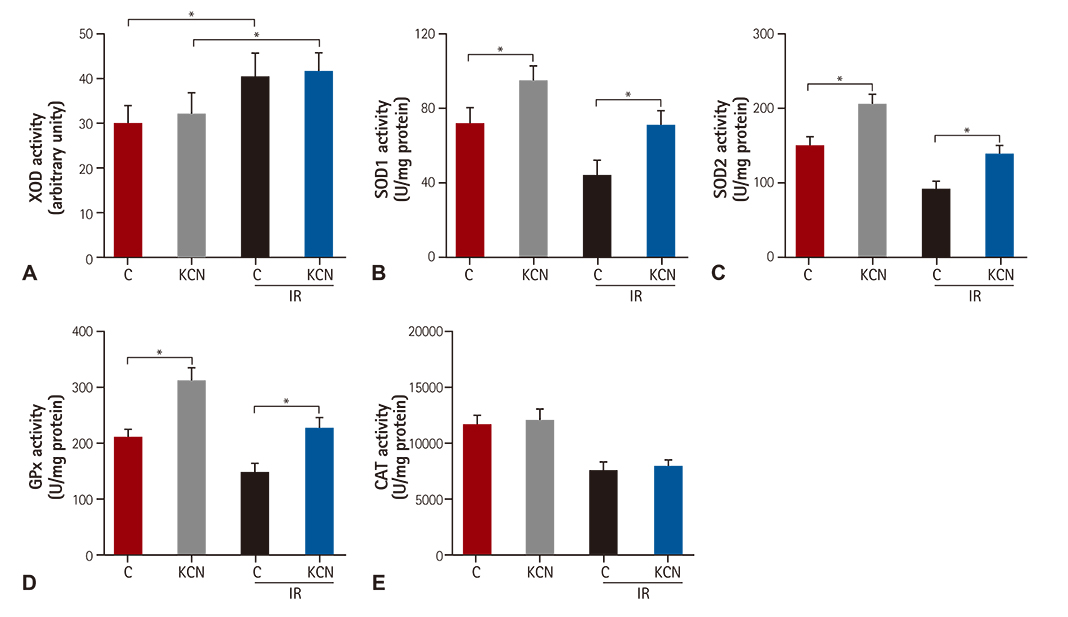
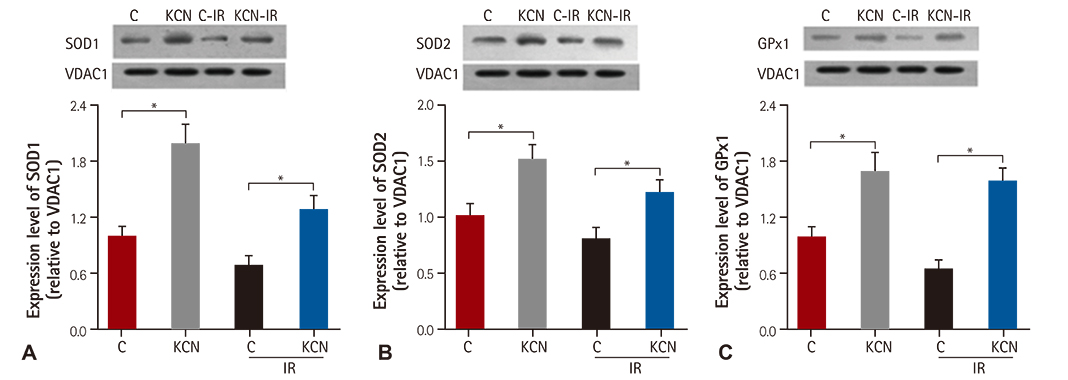
KCN pre-treatment led to decreased infarction area after IR injury and improved cardiac function. KCN pre-treated group challenged with IR injury was associated with reduced ROS production through inhibition of activity and not downregulation of CcO expression. In addition, KCN pre-treatment was associated with enhanced expression and activity of mitochondrial antioxidase, suggesting the role of CcO in regulating IR injury through oxidative stress.
CONCLUSION
KCN pre-treatment reduced the severity of IR injury. The potential mechanism could be increased endogenous anti-oxidase activity and consequently, the enhanced clearance of ROS.
MeSH Terms
-
Animals
Cytochromes c*
Cytochromes*
Down-Regulation
Electron Transport Complex IV*
Infarction
Ischemia*
Mitochondria
Myocardial Infarction
Oxidative Stress
Potassium Cyanide
Rats
Reactive Oxygen Species
Reperfusion Injury*
Cytochromes
Cytochromes c
Electron Transport Complex IV
Potassium Cyanide
Reactive Oxygen Species
Figure
Reference
-
1. Tsukihara T, Aoyama H, Yamashita E, et al. Structures of metal sites of oxidized bovine heart cytochrome c oxidase at 2.8 A. Science. 1995; 269:1069–1074.2. Hüttemann M, Helling S, Sanderson TH, et al. Regulation of mitochondrial respiration and apoptosis through cell signaling: cytochrome c oxidase and cytochrome c in ischemia/reperfusion injury and inflammation. Biochim Biophys Acta. 2012; 1817:598–609.3. Groening P, Huang Z, La Gamma EF, Levy RJ. Glutamine restores myocardial cytochrome C oxidase activity and improves cardiac function during experimental sepsis. JPEN J Parenter Enteral Nutr. 2011; 35:249–254.4. Bruno C, Martinuzzi A, Tang Y, et al. A stop-codon mutation in the human mtDNA cytochrome c oxidase I gene disrupts the functional structure of complex IV. Am J Hum Genet. 1999; 65:611–620.5. Comi GP, Bordoni A, Salani S, et al. Cytochrome c oxidase subunit I microdeletion in a patient with motor neuron disease. Ann Neurol. 1998; 43:110–116.6. Muller-Hocker J. Cytochrome-c-oxidase deficient cardiomyocytes in the human heart--an age-related phenomenon. A histochemical ultracytochemical study. Am J Pathol. 1989; 134:1167–1173.7. Antonicka H, Mattman A, Carlson CG, et al. Mutations in COX15 produce a defect in the mitochondrial heme biosynthetic pathway, causing early-onset fatal hypertrophic cardiomyopathy. Am J Hum Genet. 2003; 72:101–114.8. Papadopoulou LC, Sue CM, Davidson MM, et al. Fatal infantile cardioencephalomyopathy with COX deficiency and mutations in SCO2, a COX assembly gene. Nat Genet. 1999; 23:333–337.9. Prabu SK, Anandatheerthavarada HK, Raza H, Srinivasan S, Spear JF, Avadhani NG. Protein kinase A-mediated phosphorylation modulates cytochrome c oxidase function and augments hypoxia and myocardial ischemia-related injury. J Biol Chem. 2006; 281:2061–2070.10. Booth EA, Flint RR, Lucas KL, Knittel AK, Lucchesi BR. Estrogen protects the heart from ischemia-reperfusion injury via COX-2-derived PGI2. J Cardiovasc Pharmacol. 2008; 52:228–235.11. Vogt S, Ramzan R, Weber P, et al. Ischemic preconditioning results in an ATP-dependent inhibition of cytochrome C oxidase. Shock. 2013; 40:407–413.12. Sun WH, Liu F, Chen Y, Zhu YC. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res Commun. 2012; 421:164–169.13. Whittington HJ, Hall AR, McLaughlin CP, Hausenloy DJ, Yellon DM, Mocanu MM. Chronic metformin associated cardioprotection against infarction: not just a glucose lowering phenomenon. Cardiovasc Drugs Ther. 2013; 27:5–16.14. Burkard N, Williams T, Czolbe M, et al. Conditional overexpression of neuronal nitric oxide synthase is cardioprotective in ischemia/reperfusion. Circulation. 2010; 122:1588–1603.15. Guo W, Cheng ZY, Zhu YZ. Hydrogen sulfide and translational medicine. Acta Pharmacol Sin. 2013; 34:1284–1291.16. Zuckerbraun BS, Chin BY, Bilban M, et al. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007; 21:1099–1106.17. Jain M, Rivera S, Monclus EA, et al. Mitochondrial reactive oxygen species regulate transforming growth factor-beta signaling. J Biol Chem. 2013; 288:770–777.18. Diaz F. Cytochrome c oxidase deficiency: patients and animal models. Biochim Biophys Acta. 2010; 1802:100–110.19. Nicholson CK, Calvert JW. Hydrogen sulfide and ischemia-reperfusion injury. Pharmacol Res. 2010; 62:289–297.20. Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007; 292:C137–C147.21. Tanaka-Esposito C, Chen Q, Lesnefsky EJ. Blockade of electron transport before ischemia protects mitochondria and decreases myocardial injury during reperfusion in aged rat hearts. Transl Res. 2012; 160:207–216.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Gabexate Mesilate on the Ishemia-Reperfusion Injury in the Rabbit Liver
- Change of Plasma Xanthine Oxidase Activity by Intermittent Hepatic Ischemia-Reperfusion
- Effects of Renal Ischemia/Reperfusion on Renal Function in Rats
- Effect of Methylprednisolone on Cytochrome Oxidase and Lipid Peroxidation of the Contused Spinal Cord
- Reactive oxygen species and N-methyl-D-aspartate receptor-mediated central sensitization in hindlimb ischemia/reperfusion injury-induced neuropathic pain rats