Nat Prod Sci.
2017 Mar;23(1):53-60. 10.20307/nps.2017.23.1.53.
Ethanol Extract of Perillae Herba Enhances Pentobarbital-Induced Sleep and Non-Rapid Eye Movement (NREM) Sleep through GABA(A)-ergic Systems
- Affiliations
-
- 1College of Pharmacy, Chungbuk National University, Cheongju 361-763, Republic of Korea. kiwan@chungbuk.ac.kr
- KMID: 2376500
- DOI: http://doi.org/10.20307/nps.2017.23.1.53
Abstract
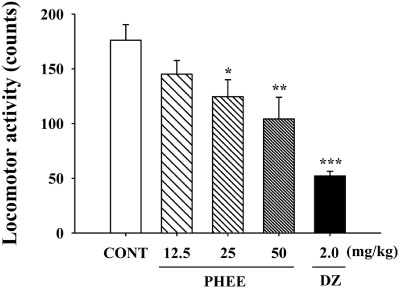
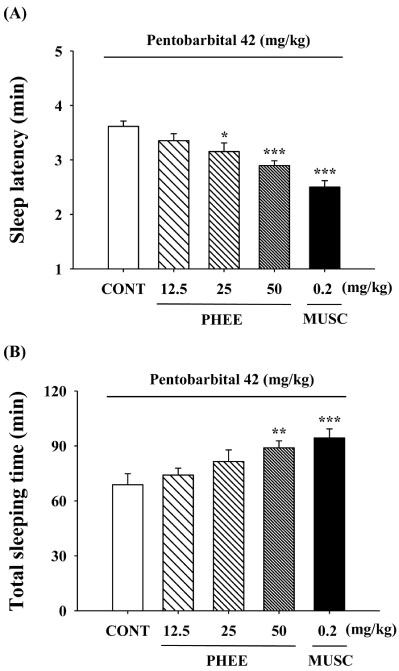
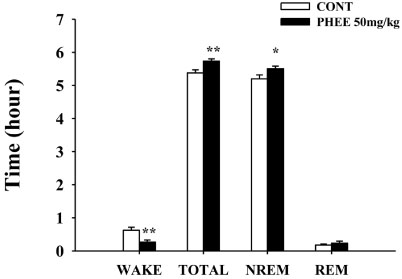
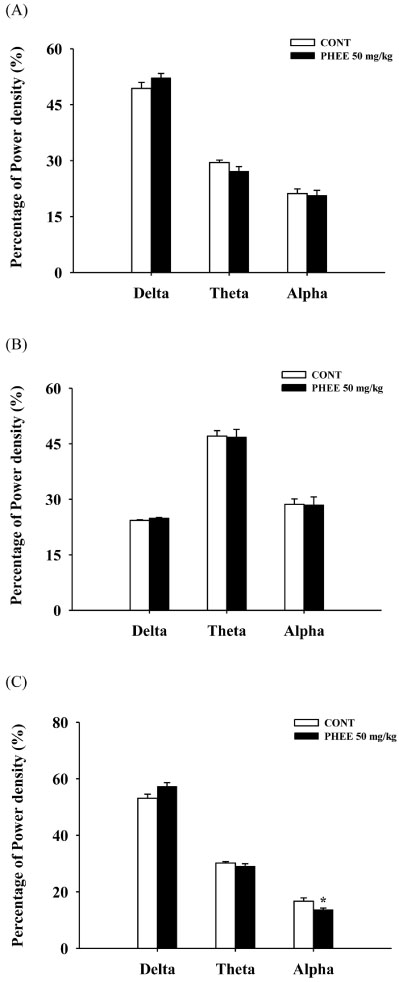
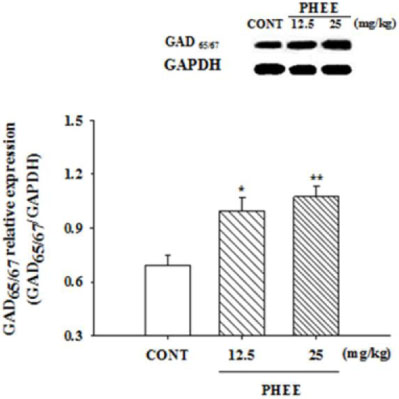
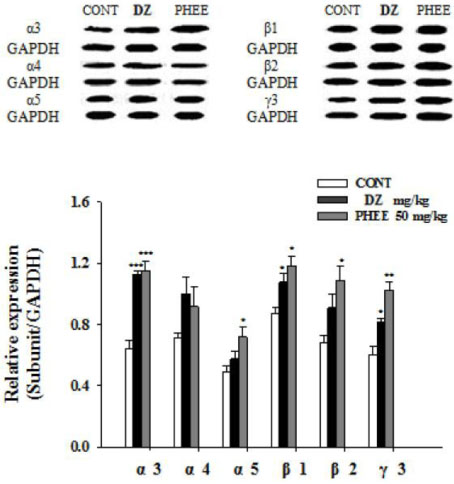
- Perillae Herba has been traditionally used for the sedation in the oriental countries. Therefore, this study was conducted to determine whether Perillae Herba ethanol extract (PHEE) enhances pentobarbital-induced sleeping behaviors in animals. In addition, the possible mechanisms are demonstrated. PHEE (12.5, 25 and 50 mg/kg. p.o.) reduced the locomotor activity in mice. PHEE reduced sleep latency and augmented the total sleep time in pentobarbital (42 mg/kg, i.p.)-induced sleep in mice. Furthermore, the number of sleeping mice treated with sub-hypnotic pentobarbital (28 mg/kg, i.p.) increased. PHEE (50 mg/kg. p.o.) decreased the sleep/wake cycles and wakefulness, and increased total sleeping time and NREM sleep in electroencephalogram (EEG) of rats. In addition, PHEE (0.1, 1.0 and 10 µg/ml) increased the intracellular Clâ» level through the GABA receptors in the hypothalamus of rats. Moreover, the protein of glutamate decarboxylase (GAD) was overexpressed by PFEE. It was found that PHEE enhanced pentobarbital-induced sleeping behaviors through GABA(A)-ergic transmissions.
Keyword
MeSH Terms
Figure
Reference
-
1. Pilcher JJ, Ginter DR, Sadowsky B. J Psychosom Res. 1997; 42:583–596.2. Doi Y, Minowa M, Tango T. Sleep. 2003; 26:467–471.3. Iliescu EA, Coo H, McMurray MH. Nephrol Dial Transplant. 2003; 18:126–132.4. Phillips KD, Sowell RL, Boyd M, Dudgeon WD, Hand GA. Mind-Body Research Group. Qual Life Res. 2005; 14:959–970.5. Joanna MW, Stachowicz K, Nowak G, Pilc A. The Loss of Glutamate-GABA Harmony in Anxiety Disorders. USA: InTech;2011. p. 24.6. Olsen RW, Sieghart W. Pharmacol Rev. 2008; 60:243–260.7. Whiting PJ. Drug Discov Today. 2003; 8:445–450.8. Ahn H. Korean J Food Preserv. 2006; 13:703–707.9. Honda G, Koezuka Y, Tabata M. Chem Pharm Bull. 1988; 36:3153–3155.10. Yi LT, Li J, Geng D. J Ethnopharmacol. 2013; 147:245–253.11. Takeda H, Tsuji M, Inazu M, Egashira T, Matsumiya T. Eur J Pharmacol. 2002; 449:261–267.12. Takeda H, Tsuji M, Miyamoto J, Matsumiya T. Psychopharmacology (Berl). 2002; 164:233–235.13. Morton GJ, Kaiyala KJ, Fisher JD, Ogimoto K, Schwartz MW, Wisse BE. Am J Physiol Endocrinol Metab. 2011; 300:E392–E401.14. Wolfman C, Viola H, Marder M, Wasowski C, Ardenghi P, Izquierdo I, Paladini AC, Medina JH. Eur J Pharmacol. 1996; 318:23–30.15. Sanford LD, Yang L, Liu X, Tang X. Brain Res. 2006; 1084:80–88.16. Tokunaga S, Takeda Y, Niimoto T, Nishida N, Kubo T, Ohno T, Matsuura Y, Kawahara Y, Shinomiya K, Kamei C. Biol Pharm Bull. 2007; 30:363–366.17. Ma Y, Han H, Eun JS, Kim HC, Hong JT, Oh KW. Biol Pharm Bull. 2007; 30:1748–1753.18. West MR, Molloy CR. Anal Biochem. 1996; 241:51–58.19. Wagner C, Vargas AP, Roos DH, Morel AF, Farina M, Nogueira CW, Aschner M, Rocha JB. Arch Toxicol. 2010; 84:89–97.20. Fanger BO. Anal Biochem. 1987; 162:11–17.21. Igarashi M, Miyazaki Y. Evid Based Complement Alternat Med. 2013; 2013:925342.22. Ito N, Nagai T, Oikawa T, Yamada H, Hanawa T. Evid Based Complement Alternat Med. 2011; 2011:512697.23. Hu HZ, Li ZW. J Physiol. 1997; 501(Pt 1):67–75.24. Möhler H. J Recept Signal Transduct Res. 2006; 26:731–740.25. Miller MA. Front Neurol. 2015; 6:1–9.26. Liu Z, Xu XH, Liu TY, Hong ZY, Urade Y, Huang ZL, Qu WM. CNS Neurosci Ther. 2012; 18:623–630.
Article27. Mehta AK, Ticku MK. Brain Res Brain Res Rev. 1999; 29:196–217.28. Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Brain Res Brain Res Rev. 2001; 37:68–80.29. Choi JJ, Kim YS, Kwon YO, Yoo JH, Chong MS, Lee MK, Hong JT, Oh KW. Nat Prod Sci. 2015; 21:219–225.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 4-Hydroxybenzaldehyde, One of Constituents from Gastrodiae Rhizoma Augments Pentobarbital-induced Sleeping Behaviors and Non-rapid Eye Movement (NREM) Sleep in Rodents
- Sinomenine, an Alkaloid Derived from Sinomenium acutum Potentiates Pentobarbital-Induced Sleep Behaviors and Non-Rapid Eye Movement (NREM) Sleep in Rodents
- Rosmarinic Acid Potentiates Pentobarbital-Induced Sleep Behaviors and Non-Rapid Eye Movement (NREM) Sleep through the Activation of GABA(A)-ergic Systems
- Poria cocos ethanol extract and its active constituent, pachymic acid, modulate sleep architectures via activation of GABA(A)-ergic transmission in rats
- Potentiation of decursinol angelate on pentobarbital-induced sleeping behaviors via the activation of GABA(A)-ergic systems in rodents