Nat Prod Sci.
2017 Mar;23(1):46-52. 10.20307/nps.2017.23.1.46.
Comprehensive Evaluation of the Anti-Helicobacter pylori Activity of Scutellariae Radix
- Affiliations
-
- 1College of Pharmacy, Sahmyook University, 815, Hwarang-ro, Nowon-gu, Seoul, 01795 Republic of Korea. sschoi@syu.ac.kr
- 2Re-creation Institute of Phytochemicals, College of Pharmacy, Sahmyook University, 815, Hwarang-ro, Nowon-gu, Seoul 01795, Republic of Korea.
- KMID: 2376499
- DOI: http://doi.org/10.20307/nps.2017.23.1.46
Abstract
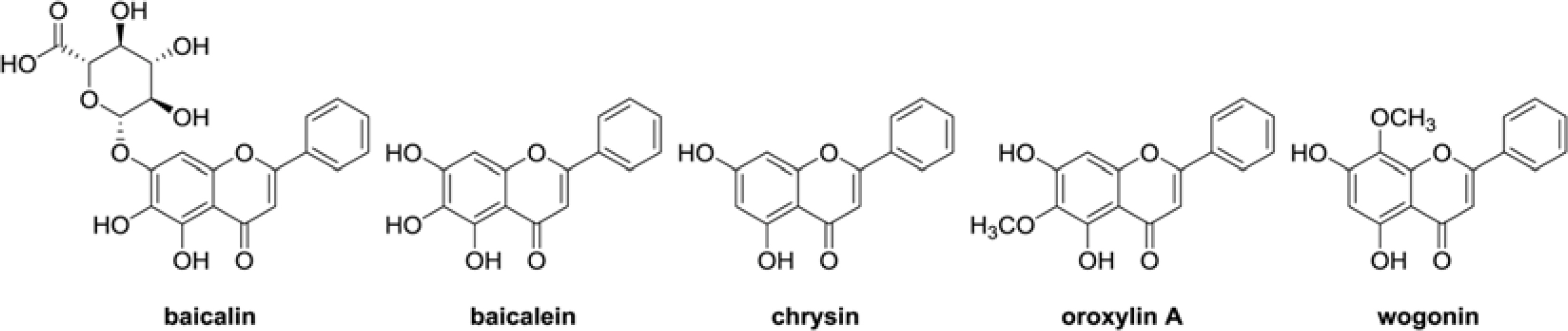
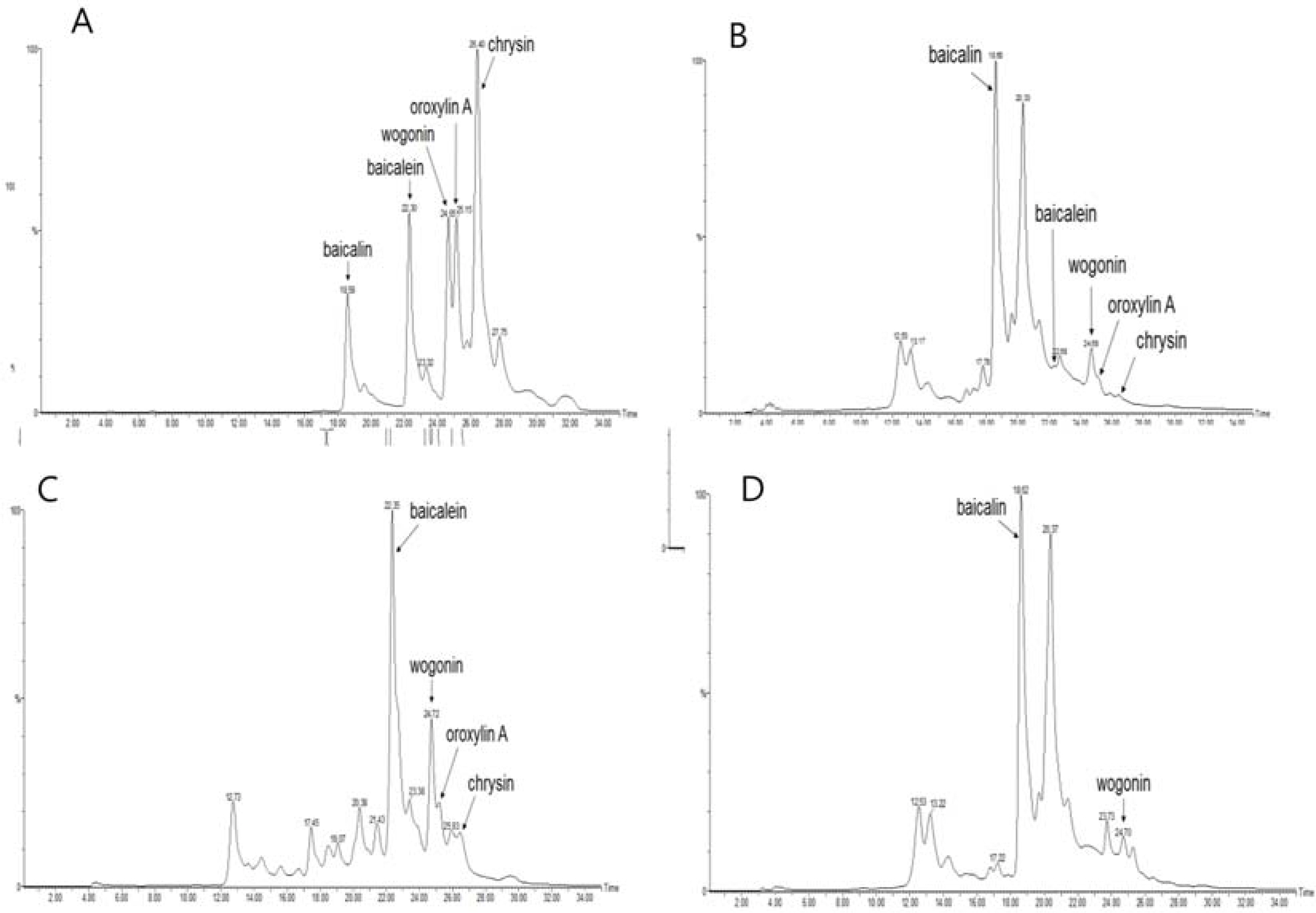
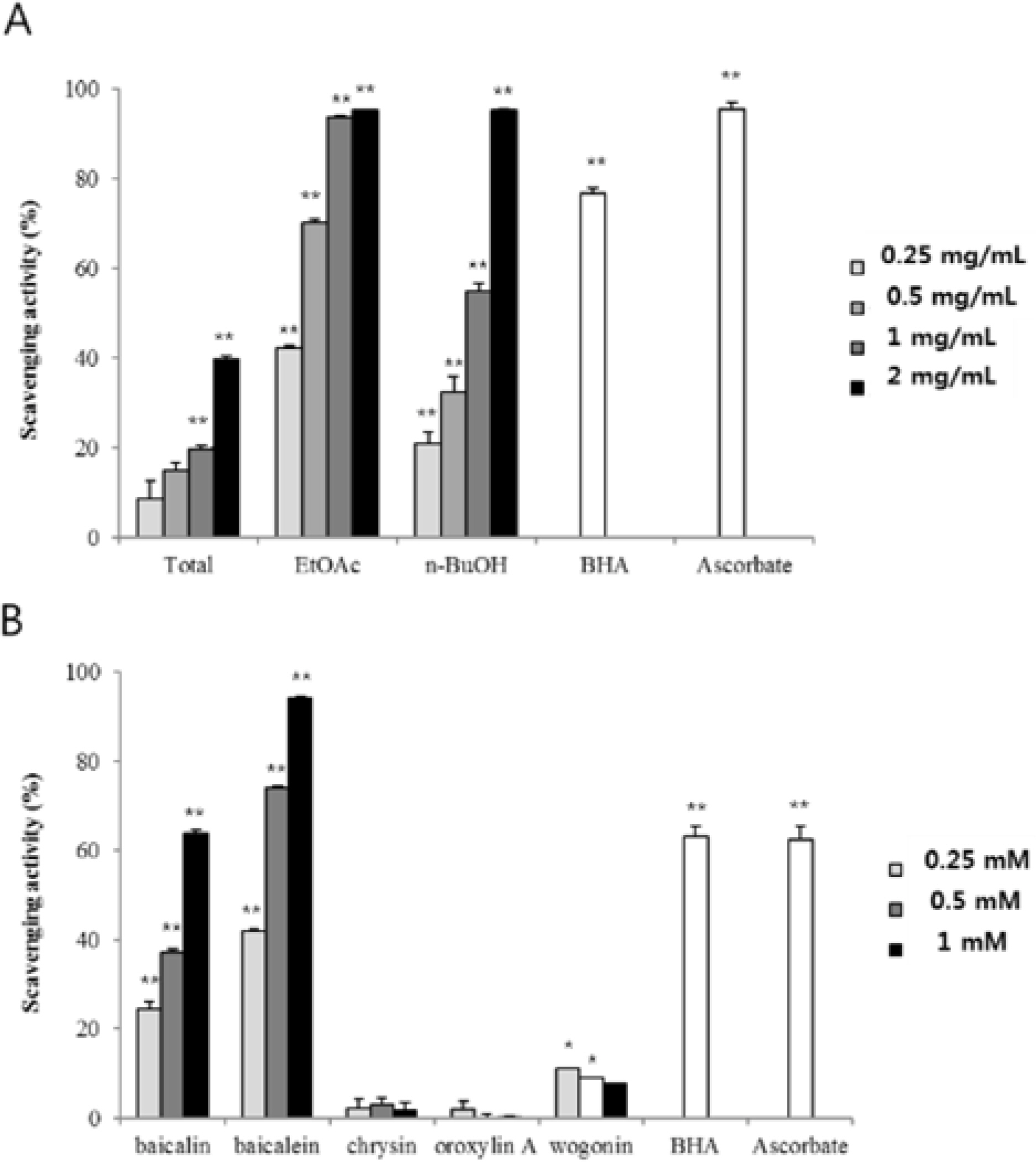
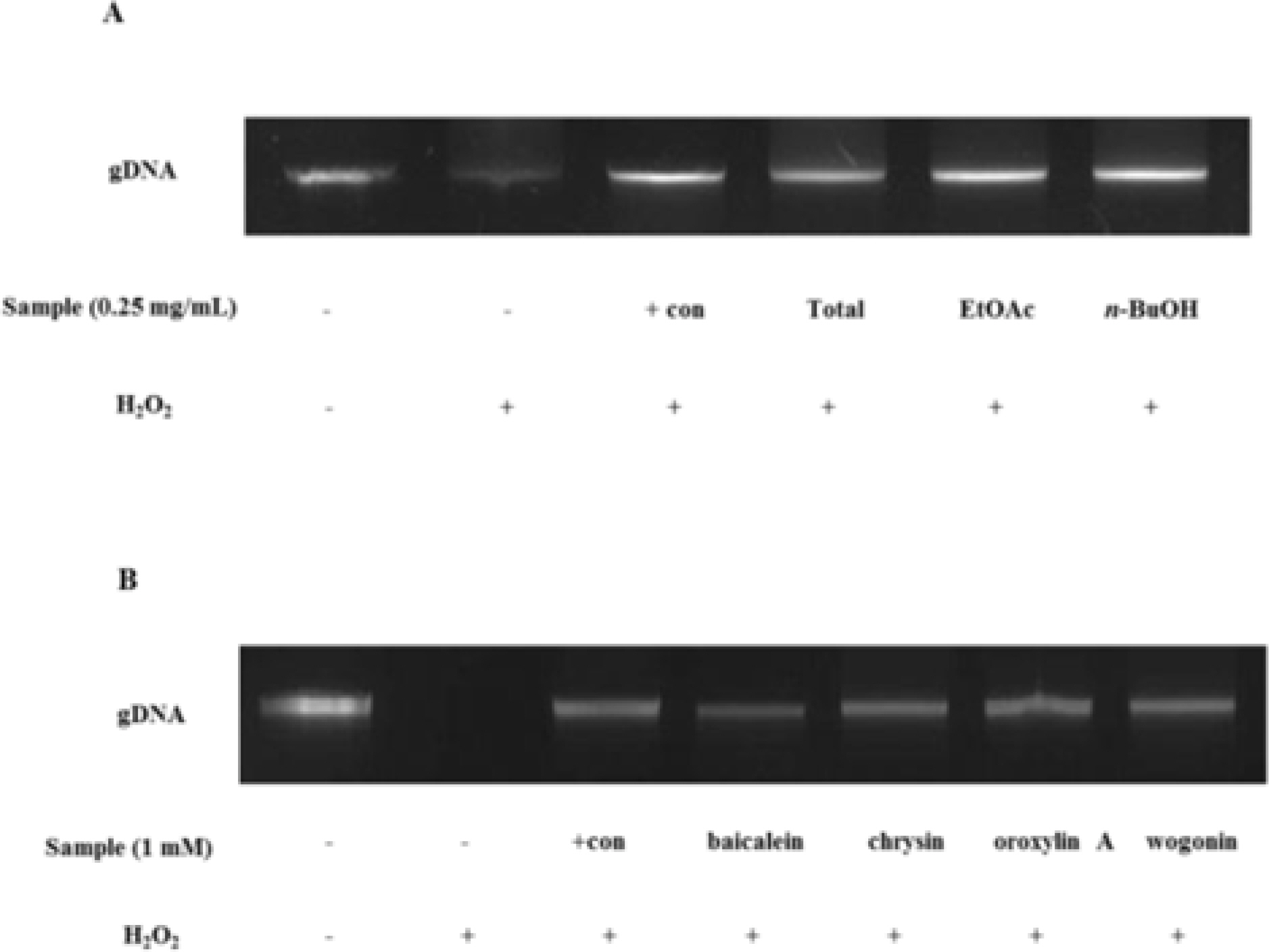
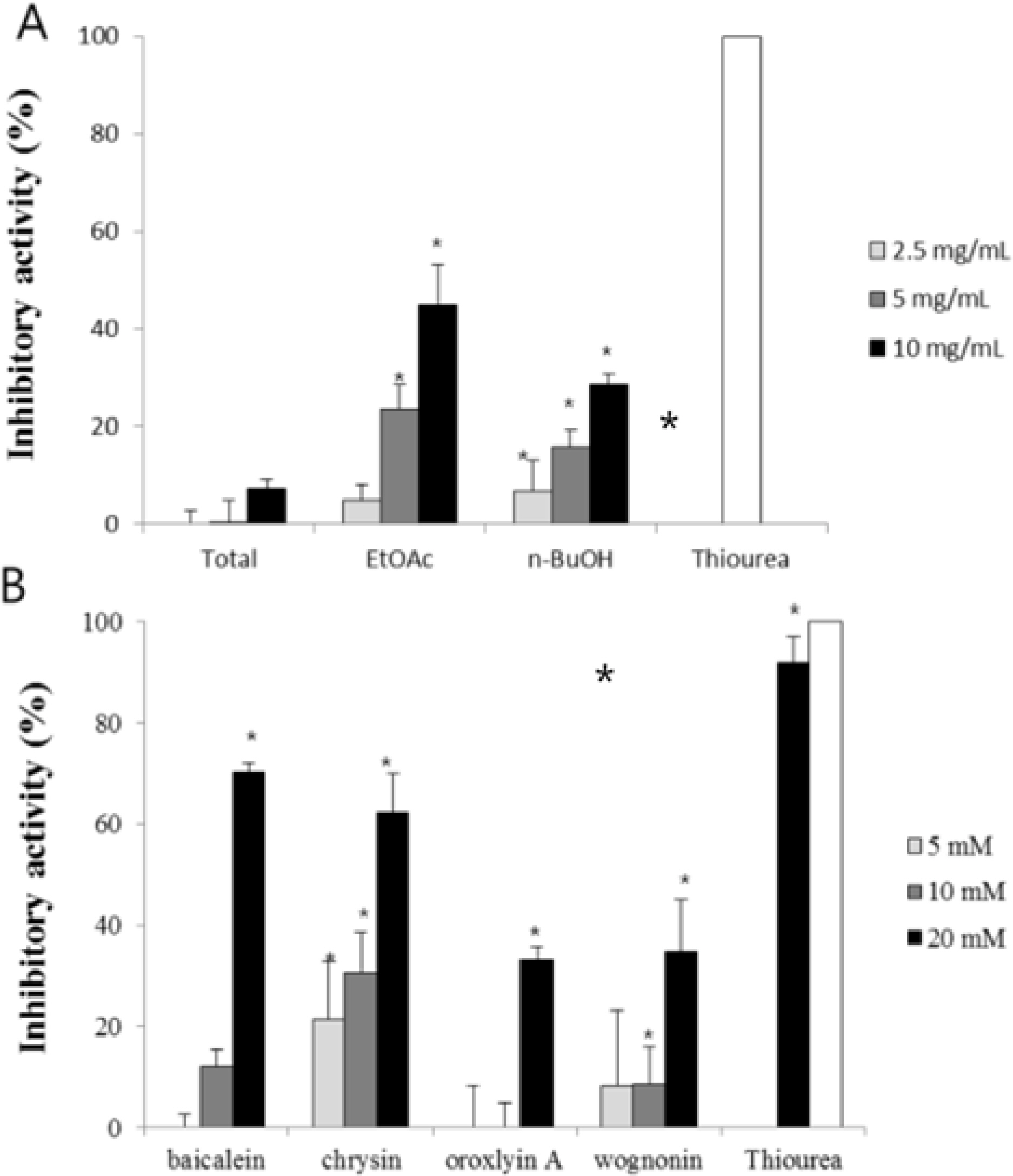
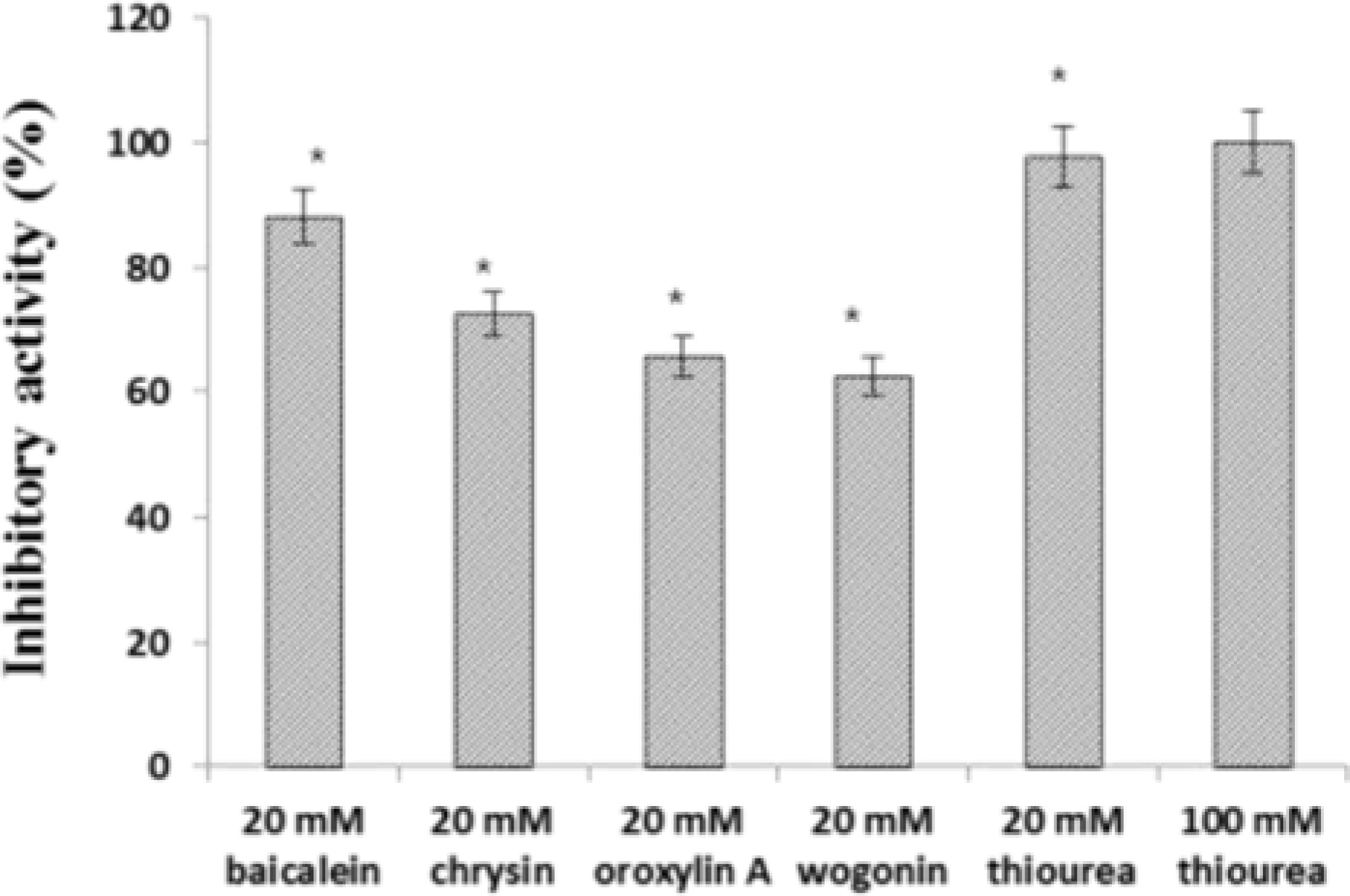
- The aim of this study was to evaluate the anti-Helicobacter pylori activity of fractions and major aglycon compounds (baicalein, chrysin, oroxylin A, wogonin) of Scutellariae Radix. Minimum inhibitory concentration (MIC) measurement, DPPH radical-scavenging assay, DNA protection assay, and urease inhibition analysis were performed. The ethyl acetate (EtOAc) fraction showed the potent anti-Helicobacter activity, and therefore, compounds in the EtOAc fraction were subjected to further assay. The MICs of chrysin, oroxylin A, and wogonin against Helicobacter pylori 26695 were 6.25, 12.5 and 25 µg/mL, respectively. Baicalein exhibited the most effective DPPH radical-scavenging activity. DNA protection using Fenton reaction, chrysin, oroxylin A, and wogonin showed effective DNA protective effect. This result was also confirmed by quantitative real-time polymerase chain reaction (qRT-PCR). Regarding Jack bean urease (0.5 mg/mL, 50 unit/mg) inhibition, 20 mM ofbaicalein and chrysin inhibited urease activity by 88.2% and 72.5%, respectively.
Keyword
MeSH Terms
Figure
Reference
-
References
(1). Peek R. M.., Blaser M. J.Nat. Rev. Cancer. 2002. 2:28–37.(2). Kim B. J.., Kim J. G.Kor. J. Med. 2015. 89:133–141.(3). De Francesco V. D.., Giorgio F.., Hassan C.., Manes G.., Vannella L.., Panella C.., Ierardi E.., Zullo A. J.Gastrointestin. Liver Dis. 2010. 19:409–414.(4). Jung K. W.., Won Y. J.., Kong H. J.., Oh C. M.., Cho H. S.., Lee D.H.., Lee K. H.Cancer Res. Treat. 2015. 47:127–141.(5). Shin A.., Park S.., Shin H. R.., Park E. H.., Park S. K.., Oh J. K.., Lim M. K.., Choi B. Y.., Boniol M.., Boffetta P.Ann. Oncol. 2011. 22:1435–1442.(6). Shin A. S.., Kim J. S.., Park S. H. J.Gastric Cancer. 2011. 11:135–140.(7). Zhang Q. B.., Nakshabendi I. M.., Mokhashi M. S.., Dawodu J. B.., Gemmell C. G.., Russell R. I.Gut. 1996. 38:841–845.(8). Shao Z. H.., Li C. Q.., Vanden Hoek T.L.., Becker L. B.., Schumacker P. T.., Wu J.A.., Attele A.S.., Yuan C. S. J.Mol. Cell. Cardiol. 1999. 31:1885–1895.(9). Huang W. H.., Lee A. R.., Yang C. H.Biosci. Biotechnol. Biochem. 2006. 70:2371–2380.(10). Wang Y. C.., Huang K. M.Food Chem. Toxicol. 2013. 53:376–383.(11). Ustün O.., Ozcellik B.., Akyön Y.., Abbasoglu U.., Yesilada E. J.Ethnopharmacol. 2006. 108:457–461.(12). Kang M. H.., Lee J. H.., Lee Y. S.., Son K. H.., Lee D. H.., Kim Y. S.., Kang S. S.., Bang H. C.., Jeong C. S.Yakhak Hoeji. 2007. 51:68–74.(13). Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, 19th Informational Supplement. 2009. . Document M100-S19, CLSI, Wayne, PA.(14). Li H. B.., Chen F. J.Chromatogr. A. 2005. 1074:107–110.(15). Choi J. S.., Oh J. I.., Hwang I. T.., Kim S. E.., Chun J. C.., Lee B. H.., Kim J. S.., Kim T. J.., Cho K. Y.Kor. J.Pestic. Sci. 2003. 7:92–99.(16). Lee J. C.., Kim H. R.., Kim J.., Jang Y. S. J.Agric. Food Chem. 2002. 50:6490–6496.(17). Weatherburn M. W.Anal. Chem. 1967. 39:971–974.(18). Choi Y. S.., Cheon J. H.., Lee J. Y.., Kim S. G.., Kim J. S.., Kim N. Y.., Lee D. H.., Kim J. M.., Jung H. C.., Song I. S.Korean J. Gastroenterol. 2006. 48:156–161.(19). Wu J.., Hu D.., Wang K. X.Zhong Yao Cai. 2008. 31:707–710.(20). Shin S. J.., Park C. E.., Baek N. I.., Chung I. S.., Park C. H.Biotechnol. Bioprocess Eng. 2009. 14:140–145.(21). Park C. E.., Park C. H.Korean Chem. Eng. Res. 2013. 51:591–596.(22). Tan L.., Su J.., Wu D.., Yu X.., Su Z.., He J.., Wu X.., Kong S.., Lai X.., Lin J.., Su Z.Scientific World Journal. 2013. doi: 10.1155/2013/879501.(23). Yu X. D.., Zheng R. B.., Xie J. H.., Su J. Y.., Huang X. Q.., Wang Y. H.., Zheng Y. F.., Mo Z. Z.., Wu X. L.., Wu D. W.., Liang Y. E.., Zeng H. F.., Su Z. R.., Huang P. J.Ethnopharmacol. 2015. 162:69–78.(24). Wu D. W.., Yu X. D.., Xie J. H.., Su Z. Q.., Su J. Y.., Tan L. R.., Huang X. Q.., Chen J. N.., Su Z. R.Fitoterapia. 2013. 91:60–67.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on the antimicrobial activities of herbal extracts against Helicobacter pylori
- Anti-Helicobacter pylori Activity of Compounds Isolated from Fraxinus mandshurica Bark
- Response to Treatment of Helicobacter pylori-associated Dyspepsia: Eradication of Helicobacter pylori or Correction of Gastric or Intestinal Dysbiosis?
- In vitro and in vivo antibacterial activity of Meliae fructus extract against Helicobacter pylori
- The Effect of Probiotics for Helicobacter pylori