Infect Chemother.
2017 Mar;49(1):62-67. 10.3947/ic.2017.49.1.62.
Impact of Revised Broad-Spectrum Cephalosporin Clinical and Laboratory Standards Institute Breakpoints on Susceptibility in Enterobacteriaceae producing AmpC β-Lactamase
- Affiliations
-
- 1Division of Infectious Diseases, Department of Internal Medicine, Kyung Hee University Hospital, Kyung Hee University School of Medicine, Seoul, Korea.
- 2Department of Infectious Diseases, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. sangho@amc.seoul.kr
- 3Department of Laboratory Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2375101
- DOI: http://doi.org/10.3947/ic.2017.49.1.62
Abstract
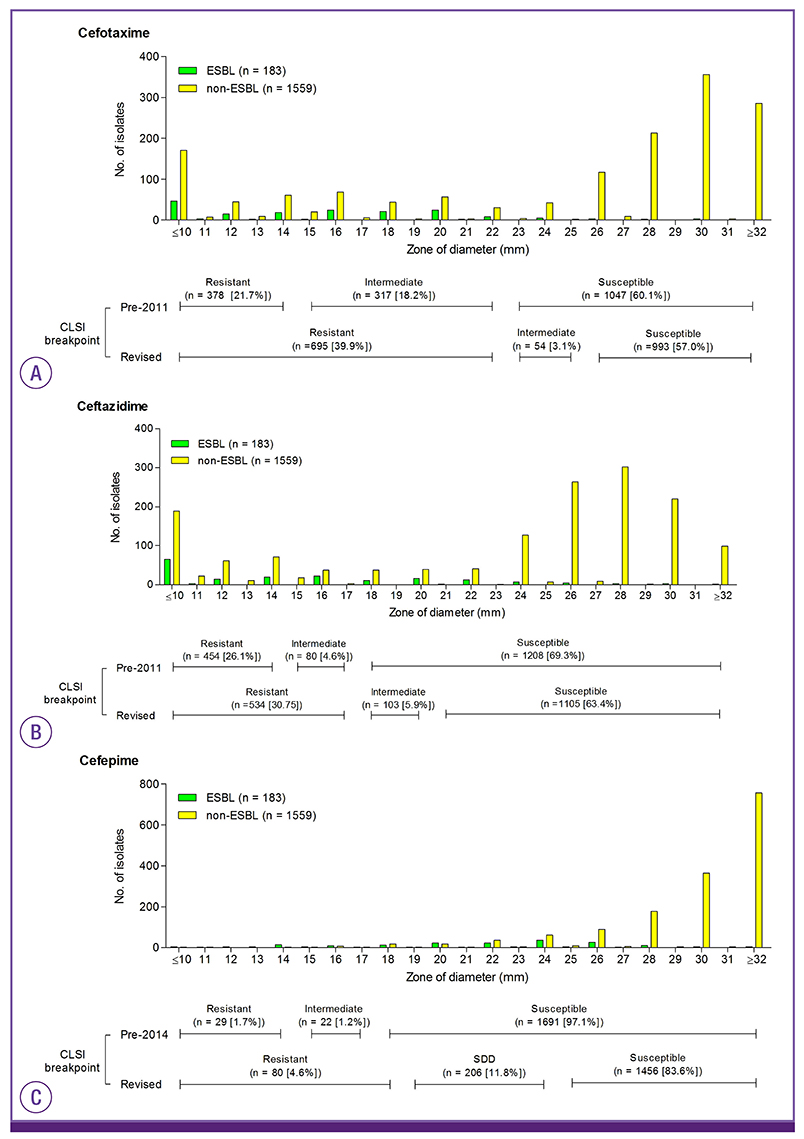
- We evaluated the impact of revised Clinical and Laboratory Standards Institute (CLSI) breakpoints for broad-spectrum cephalosporins (BSCs) on the susceptibilities of 1,742 isolates of Enterobacter species, Serratia marcescens, Citrobacter freundii, and Morganella morganii. The 2011 CLSI criteria for cefotaxime and ceftazidime reduced the rates of susceptibility by 2.9% and 5.9%, respectively. The 2014 CLSI criteria for cefepime reduced the rate of susceptibility by 13.9%, and categorized 11.8% isolates as susceptible-dose dependent (SDD) for cefepime. Among 183 isolates with extended-spectrum ß-lactamase (ESBL) phenotype, implementation of the new criteria reduced the rates of susceptibility to cefotaxime, ceftazidime, and cefepime by 2.8%, 14.8%, and 53.6%, respectively. The proportion of ESBL phenotype among BSC-susceptible isolates was low (0.9% for cefotaxime, 3.0% for ceftazidime, and 3.3% for cefepime). In summary, implementation of new CLSI criteria led to little change in susceptibility to cefotaxime and ceftazidime but a substantial change in susceptibility to cefepime. The recognition of revised CLSI criteria for BSC and SDD will help clinicians to select the optimal antibiotic and dosing regimen.
Keyword
MeSH Terms
Figure
Reference
-
1. Clinical and Laboratory Standards Institue (CLSI). Performance standards for antimicrobial susceptibility resting: 21th infomational supplment CLSI document M100-S21. Wayne, PA: CLSI;2011.2. Clinical and Laboratory Standards Institue (CLSI). Performance standards for antimicrobial susceptibility resting: 24th infomational supplment CLSI document M100-S24. Wayne, PA: CLSI;2014.3. Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN; Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute. Background and rationale for revised clinical and laboratory standards institute interpretive criteria (breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. cephalosporins and aztreonam. Clin Infect Dis. 2013; 56:1301–1309.
Article4. Sanders WE Jr, Sanders CC. Enterobacter spp.: pathogens poised to flourish at the turn of the century. Clin Microbiol Rev. 1997; 10:220–241.
Article5. Choi SH, Lee JE, Park SJ, Kim MN, Choo EJ, Kwak YG, Jeong JY, Woo JH, Kim NJ, Kim YS. Prevalence, microbiology, and clinical characteristics of extended-spectrum beta-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganii in Korea. Eur J Clin Microbiol Infect Dis. 2007; 26:557–561.
Article6. Choi SH, Lee JE, Park SJ, Choi SH, Lee SO, Jeong JY, Kim MN, Woo JH, Kim YS. Emergence of antibiotic resistance during therapy for infections caused by Enterobacteriaceae producing AmpC beta-lactamase: implications for antibiotic use. Antimicrob Agents Chemother. 2008; 52:995–1000.
Article7. Towne TG, Lewis JS 2nd, Herrera M, Wickes B, Jorgensen JH. Detection of SHV-type extended-spectrum beta-lactamase in Enterobacter isolates. J Clin Microbiol. 2010; 48:298–299.
Article8. Stürenburg E, Sobottka I, Noor D, Laufs R, Mack D. Evaluation of a new cefepime-clavulanate ESBL Etest to detect extended-spectrum beta-lactamases in an Enterobacteriaceae strain collection. J Antimicrob Chemother. 2004; 54:134–138.9. Jacoby GA, Han P. Detection of extended-spectrum beta-lactamases in clinical isolates of Klebsiella pneumoniae and Escherichia coli . J Clin Microbiol. 1996; 34:908–911.
Article10. Andes D, Craig WA. Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect. 2005; 11:Suppl 6. 10–17.
Article11. Kohner PC, Robberts FJ, Cockerill FR 3rd, Patel R. Cephalosporin MIC distribution of extended-spectrum-β-lactamase- and pAmpC-producing Escherichia coli and Klebsiella species. J Clin Microbiol. 2009; 47:2419–2425.
Article12. Hamada Y, Sutherland CA, Nicolau DP. Impact of revised cefepime CLSI breakpoints on Escherichia coli and Klebsiella pneumoniae susceptibility and potential impact if applied to Pseudomonas aeruginosa . J Clin Microbiol. 2015; 53:1712–1714.
Article13. Lee NY, Lee CC, Li CW, Li MC, Chen PL, Chang CM, Ko WC. Cefepime therapy for monomicrobial Enterobacter cloacae bacteremia: Unfavorable outcomes in patients infected by cefepime-susceptible dose-dependent isolates. Antimicrob Agents Chemother. 2015; 59:7558–7563.
Article14. Lee NY, Lee CC, Huang WH, Tsui KC, Hsueh PR, Ko WC. Cefepime therapy for monomicrobial bacteremia caused by cefepime-susceptible extended-spectrum beta-lactamase-producing Enterobacteriaceae: MIC matters. Clin Infect Dis. 2013; 56:488–495.
Article15. Kim SJ, Park KH, Chung JW, Sung H, Choi SH, Choi SH. Prevalence and impact of extended-spectrum beta-lactamase production on clinical outcomes in cancer patients with Enterobacter species bacteremia. Korean J Intern Med. 2014; 29:637–646.
Article16. Cheong HS, Ko KS, Kang CI, Chung DR, Peck KR, Song JH. Clinical significance of infections caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae blood isolates with inducible AmpC beta-lactamase. Microb Drug Resist. 2012; 18:446–452.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Clinical and Laboratory Standards Institute and European Committee on Antimicrobial Susceptibility Testing Breakpoints for β-Lactams in Enterobacteriaceae Producing Extended-Spectrum β-Lactamases and/or Plasmid-Mediated AmpC β-Lactamases
- Pitfalls of the Clinical and Laboratory Standards Institute’s Revised Breakpoints on Interpretation of the Cephalosporin Susceptibility of an Extended-Spectrum β-lactamase Producing Klebsiella pneumoniae: Analysis of a 2010 Nationwide Proficiency Survey
- Introduction to the revised international guidelines on breakpoints for antimicrobial susceptibility testing
- EUCAST v.12.0 review focused on changes
- Strategies for Interpretive Standards of beta-Lactams Susceptibility Testing and Identification of Extended-Spectrum beta-Lactamases and Carbapenemases in Enterobacteriaceae