Yonsei Med J.
2016 Jul;57(4):865-871. 10.3349/ymj.2016.57.4.865.
Metabolic Pathway Signatures Associated with Urinary Metabolite Biomarkers Differentiate Bladder Cancer Patients from Healthy Controls
- Affiliations
-
- 1Department of Urology, Chungbuk National University College of Medicine, Cheongju, Korea. wjkim@chungbuk.ac.kr
- 2Department of Urology, Graduate School of Medicine, Yonsei University, Seoul, Korea.
- 3College of Pharmacy, Natural Product Research Institute, Seoul National University, Seoul, Korea.
- 4School of Food Science and Technology, Chung-Ang University, Anseong, Korea.
- 5Department of Biochemistry, Dongeui University College of Oriental Medicine, Busan, Korea.
- 6Section of Urological Oncology, The Cancer Institute of New Jersey, Robert Wood Johnson Medical School, New Brunswick, NJ, USA.
- 7Department of Surgery, Harvard Medical School, Boston, MA, USA. Jayoung.Kim@cshs.org
- 8Cancer Biology Division, Departments of Surgery and Biomedical Sciences, Cedars-Sinai Medical Center, Los Angeles, CA, USA.
- KMID: 2374116
- DOI: http://doi.org/10.3349/ymj.2016.57.4.865
Abstract
- PURPOSE
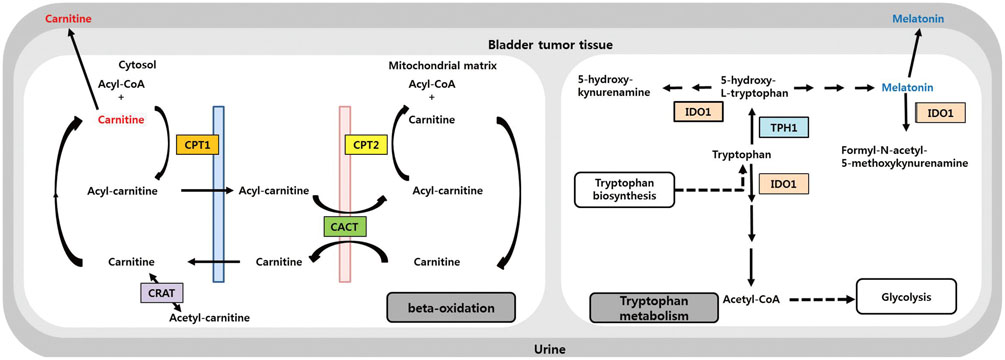
Our previous high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry study identified bladder cancer (BCA)-specific urine metabolites, including carnitine, acylcarnitines, and melatonin. The objective of the current study was to determine which metabolic pathways are perturbed in BCA, based on our previously identified urinary metabolome.
MATERIALS AND METHODS
A total of 135 primary BCA samples and 26 control tissue samples from healthy volunteers were analyzed. The association between specific urinary metabolites and their related encoding genes was analyzed.
RESULTS
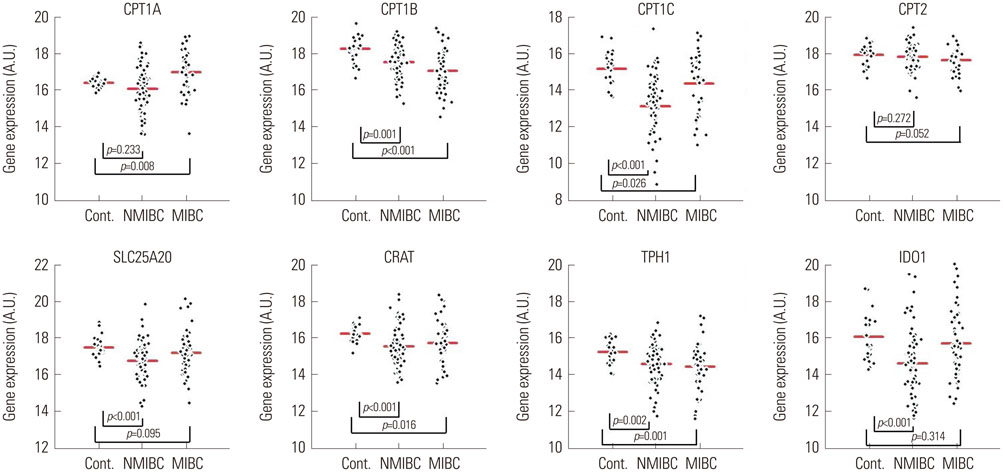
Significant alterations in the carnitine-acylcarnitine and tryptophan metabolic pathways were detected in urine specimens from BCA patients compared to those of healthy controls. The expression of eight genes involved in the carnitine-acylcarnitine metabolic pathway (CPT1A, CPT1B, CPT1C, CPT2, SLC25A20, and CRAT) or tryptophan metabolism (TPH1 and IDO1) was assessed by RT-PCR in our BCA cohort (n=135). CPT1B, CPT1C, SLC25A20, CRAT, TPH1, and IOD1 were significantly downregulated in tumor tissues compared to normal bladder tissues (p<0.05 all) of patients with non-muscle invasive BCA, whereas CPT1B, CPT1C, CRAT, and TPH1 were downregulated in those with muscle invasive BCA (p<0.05), with no changes in IDO1 expression.
CONCLUSION
Alterations in the expression of genes associated with the carnitine-acylcarnitine and tryptophan metabolic pathways, which were the most perturbed pathways in BCA, were determined.
MeSH Terms
-
Aged
Biomarkers/metabolism
Carcinoma, Transitional Cell/genetics/*metabolism/pathology
Carnitine/*analogs & derivatives/genetics/metabolism
Case-Control Studies
Female
Humans
Male
Metabolic Networks and Pathways/*physiology
Middle Aged
RNA, Messenger/metabolism
Real-Time Polymerase Chain Reaction
Urinary Bladder Neoplasms/genetics/*metabolism/pathology
Biomarkers
Carnitine
RNA, Messenger
Figure
Reference
-
1. Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013; 63:234–241.
Article2. van der Aa MN, Steyerberg EW, Sen EF, Zwarthoff EC, Kirkels WJ, van der Kwast TH, et al. Patients' perceived burden of cystoscopic and urinary surveillance of bladder cancer: a randomized comparison. BJU Int. 2008; 101:1106–1110.
Article3. Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003; 61:109–118.
Article4. Putluri N, Shojaie A, Vasu VT, Vareed SK, Nalluri S, Putluri V, et al. Metabolomic profiling reveals potential markers and bioprocesses altered in bladder cancer progression. Cancer Res. 2011; 71:7376–7386.
Article5. Liu X, Zhang L, You L, Yu J, Zhao J, Li L, et al. Differential toxicological effects induced by mercury in gills from three pedigrees of Manila clam Ruditapes philippinarum by NMR-based metabolomics. Ecotoxicology. 2011; 20:177–186.
Article6. Xu XJ, Zheng P, Ren GP, Liu ML, Mu J, Guo J, et al. 2,4-Dihydroxypyrimidine is a potential urinary metabolite biomarker for diagnosing bipolar disorder. Mol Biosyst. 2014; 10:813–819.
Article7. Garcia E, Andrews C, Hua J, Kim HL, Sukumaran DK, Szyperski T, et al. Diagnosis of early stage ovarian cancer by 1H NMR metabonomics of serum explored by use of a microflow NMR probe. J Proteome Res. 2011; 10:1765–1771.
Article8. OuYang D, Xu J, Huang H, Chen Z. Metabolomic profiling of serum from human pancreatic cancer patients using 1H NMR spectroscopy and principal component analysis. Appl Biochem Biotechnol. 2011; 165:148–154.
Article9. Mitra AP, Bartsch CC, Cote RJ. Strategies for molecular expression profiling in bladder cancer. Cancer Metastasis Rev. 2009; 28:317–326.
Article10. Alberice JV, Amaral AF, Armitage EG, Lorente JA, Algaba F, Carrilho E, et al. Searching for urine biomarkers of bladder cancer recurrence using a liquid chromatography-mass spectrometry and capillary electrophoresis-mass spectrometry metabolomics approach. J Chromatogr A. 2013; 1318:163–170.
Article11. Huang Z, Lin L, Gao Y, Chen Y, Yan X, Xing J, et al. Bladder cancer determination via two urinary metabolites: a biomarker pattern approach. Mol Cell Proteomics. 2011; 10:M111.007922.
Article12. Pasikanti KK, Esuvaranathan K, Hong Y, Ho PC, Mahendran R, Raman Nee Mani L, et al. Urinary metabotyping of bladder cancer using two-dimensional gas chromatography time-of-flight mass spectrometry. J Proteome Res. 2013; 12:3865–3873.
Article13. Jin X, Yun SJ, Jeong P, Kim IY, Kim WJ, Park S. Diagnosis of bladder cancer and prediction of survival by urinary metabolomics. Oncotarget. 2014; 5:1635–1645.
Article14. Rebouche CJ. Carnitine. In : Shils ME, Olson JA, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease. 9th ed. New York: Lippincott Williams and Wilkins;1999. p. 505–512.15. Jogl G, Tong L. Crystal structure of carnitine acetyltransferase and implications for the catalytic mechanism and fatty acid transport. Cell. 2003; 112:113–122.
Article16. Reiter RJ, Tan DX, Terron MP, Flores LJ, Czarnocki Z. Melatonin and its metabolites: new findings regarding their production and their radical scavenging actions. Acta Biochim Pol. 2007; 54:1–9.
Article17. Babjuk M, Oosterlinck W, Sylvester R, Kaasinen E, Böhle A, Palou-Redorta J. European Association of Urology (EAU). AU guidelines on non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2008; 54:303–314.18. Greene FL. The American Joint Committee on Cancer: updating the strategies in cancer staging. Bull Am Coll Surg. 2002; 87:13–15.19. Kim WT, Yun SJ, Park C, Kim IY, Moon SK, Kwon TG, et al. Identification of C16orf74 as a marker of progression in primary non-muscle invasive bladder cancer. PLoS One. 2010; 5:e15260.
Article20. van der Leij FR, Huijkman NC, Boomsma C, Kuipers JR, Bartelds B. Genomics of the human carnitine acyltransferase genes. Mol Genet Metab. 2000; 71:139–153.
Article21. Murthy MS, Pande SV. Mechanism of carnitine acylcarnitine translocase-catalyzed import of acylcarnitines into mitochondria. J Biol Chem. 1984; 259:9082–9089.
Article22. Bieber LL. Carnitine. Annu Rev Biochem. 1988; 57:261–283.
Article23. McKinney J, Teigen K, Frøystein NA, Salaün C, Knappskog PM, Haavik J, et al. Conformation of the substrate and pterin cofactor bound to human tryptophan hydroxylase. Important role of Phe313 in substrate specificity. Biochemistry. 2001; 40:15591–15601.
Article24. Najfeld V, Menninger J, Muhleman D, Comings DE, Gupta SL. Localization of indoleamine 2,3-dioxygenase gene (INDO) to chromosome 8p12-->p11 by fluorescent in situ hybridization. Cytogenet Cell Genet. 1993; 64:231–232.
Article25. Bryan GT. The role of urinary tryptophan metabolites in the etiology of bladder cancer. Am J Clin Nutr. 1971; 24:841–847.
Article26. Bansal N, Gupta A, Mitash N, Shakya PS, Mandhani A, Mahdi AA, et al. Low- and high-grade bladder cancer determination via human serum-based metabolomics approach. J Proteome Res. 2013; 12:5839–5850.
Article27. Tripathi P, Somashekar BS, Ponnusamy M, Gursky A, Dailey S, Kunju P, et al. HR-MAS NMR tissue metabolomic signatures cross-validated by mass spectrometry distinguish bladder cancer from benign disease. J Proteome Res. 2013; 12:3519–3528.
Article28. Wu XR. Biology of urothelial tumorigenesis: insights from genetically engineered mice. Cancer Metastasis Rev. 2009; 28:281–290.
Article29. Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009; 137:87–98.
Article30. Shariat SF, Kim JH, Andrews B, Kattan MW, Wheeler TM, Kim IY, et al. Preoperative plasma levels of transforming growth factor beta(1) strongly predict clinical outcome in patients with bladder carcinoma. Cancer. 2001; 92:2985–2992.
Article31. Derdak Z, Villegas KA, Harb R, Wu AM, Sousa A, Wands JR. Inhibition of p53 attenuates steatosis and liver injury in a mouse model of non-alcoholic fatty liver disease. J Hepatol. 2013; 58:785–791.
Article32. Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, et al. p53 regulates mitochondrial respiration. Science. 2006; 312:1650–1653.
Article33. Muller AJ, DuHadaway JB, Donover PS, Sutanto-Ward E, Prendergast GC. Inhibition of indoleamine 2,3-dioxygenase, an immunoregulatory target of the cancer suppression gene Bin1, potentiates cancer chemotherapy. Nat Med. 2005; 11:312–319.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Challenges in Metabolite Biomarkers as Avenues of Diagnosis and Prognosis of Cancer
- Biomarkers for bladder cancer: The search continues!
- Pilot Study of the Clinical Significance of Serum and Urinary HER-2/neu Protein in Bladder Cancer Patients
- Advances in urinary biomarker discovery in urological research
- Urinary Metabolites as Biomarkers for Diagnosis of Breast Cancer: A Preliminary Study