J Gastric Cancer.
2014 Sep;14(3):196-203. 10.5230/jgc.2014.14.3.196.
Expression Levels of Vascular Endothelial Growth Factors A and C in Patients with Peptic Ulcers and Gastric Cancer
- Affiliations
-
- 1Department of Immunology, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
- 2Molecular and Cell Biology Research Center, Mazandaran University of Medical Sciences, Sari, Iran. ajami36@gmail.com
- 3Department of Immunology, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
- 4Faculty of Advanced Medical Science Technology, Golestan University of Medical Sciences, Gorgan, Iran.
- 5Inflammatory Diseases of Upper GI Tract Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
- 6Department of Pathology, Islamic Azad University, Sari Branch, Sari, Iran.
- 7Department of Immunology, Faculty of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
- KMID: 2372531
- DOI: http://doi.org/10.5230/jgc.2014.14.3.196
Abstract
- PURPOSE
Vascular endothelial growth factor (VEGF) is one of the most important growth factors for metastatic tumors. To clarify the role of VEGF-A and C in patients with peptic ulcer disease (PUD) or gastric cancer (GC), we evaluated the expression levels of these two molecules. We also analyzed the effect of Helicobacter pylori infection on VEGF-A and C expression levels.
MATERIALS AND METHODS
Patients with dyspepsia who needed diagnostic endoscopy were selected and divided into three groups: non-ulcer dyspepsia (NUD), PUD, and GC, according to their endoscopic and histopathological results. Fifty-two patients with NUD, 50 with PUD, and 38 with GC were enrolled in this study. H. pylori infection was diagnosed by the rapid urease test. After RNA extraction and synthesis of cDNA, the expression levels of VEGF-A and C were determined by quantitative reverse transcriptase polymerase chain reaction.
RESULTS
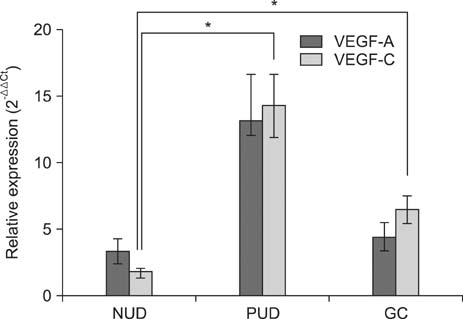
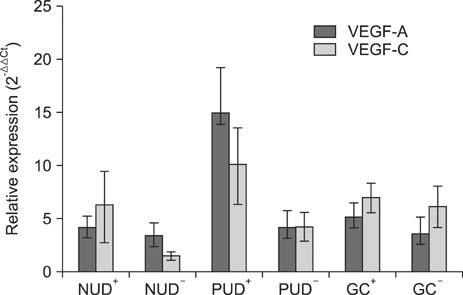
The VEGF-C expression level in the PUD and GC groups was significantly higher than that in the NUD group. Moreover, the VEGF-A expression level in the PUD and GC groups was higher than in the NUD group, although the differences were not statistically significant. Significant positive correlations were also observed between the expression levels of these two molecules in the PUD and GC groups. In addition, the expression levels of these two molecules were higher in H. pylori positive patients with PUD or GC than in H. pylori negative patients of the same groups; however, these differences did not reach statistical significance.
CONCLUSIONS
Up-regulation of VEGF-C expression during gastric mucosal inflammation may play a role in the development of peptic ulcers or GC.
Keyword
MeSH Terms
-
DNA, Complementary
Dyspepsia
Endoscopy
Helicobacter pylori
Humans
Inflammation
Intercellular Signaling Peptides and Proteins
Peptic Ulcer*
Reverse Transcriptase Polymerase Chain Reaction
RNA
Stomach Neoplasms*
Up-Regulation
Urease
Vascular Endothelial Growth Factor A*
Vascular Endothelial Growth Factor C
Vascular Endothelial Growth Factors*
DNA, Complementary
Intercellular Signaling Peptides and Proteins
RNA
Urease
Vascular Endothelial Growth Factor A
Vascular Endothelial Growth Factor C
Vascular Endothelial Growth Factors
Figure
Reference
-
1. Selgrad M, Bornschein J, Rokkas T, Malfertheiner P. Clinical aspects of gastric cancer and Helicobacter pylori--screening, prevention, and treatment. Helicobacter. 2010; 15:Suppl 1. 40–45.
Article2. Mokrowiecka A, Jurek K, Pińkowski D, Małecka-Panas E. The comparison of Health-Related Quality of Life (HRQL) in patients with GERD, peptic ulcer disease and ulcerative colitis. Adv Med Sci. 2006; 51:142–147.3. Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005; 128:1567–1578.
Article4. Rad R, Dossumbekova A, Neu B, Lang R, Bauer S, Saur D, et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004; 53:1082–1089.
Article5. Portal-Celhay C, Perez-Perez GI. Immune responses to Helicobacter pylori colonization: mechanisms and clinical outcomes. Clin Sci (Lond). 2006; 110:305–314.
Article6. Amieva MR, El-Omar EM. Host-bacterial interactions in Helicobacter pylori infection. Gastroenterology. 2008; 134:306–323.
Article7. McColm JR, Geisen P, Hartnett ME. VEGF isoforms and their expression after a single episode of hypoxia or repeated fluctuations between hyperoxia and hypoxia: relevance to clinical ROP. Mol Vis. 2004; 10:512–520.8. Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004; 56:549–580.
Article9. de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol. 2006; 177:7322–7331.
Article10. Yuanming L, Feng G, Lei T, Ying W. Quantitative analysis of lymphangiogenic markers in human gastroenteric tumor. Arch Med Res. 2007; 38:106–112.
Article11. Zhang H, Wu J, Meng L, Shou CC. Expression of vascular endothelial growth factor and its receptors KDR and Flt-1 in gastric cancer cells. World J Gastroenterol. 2002; 8:994–998.
Article12. Liu XE, Sun XD, Wu JM. Expression and significance of VEGF-C and FLT-4 in gastric cancer. World J Gastroenterol. 2004; 10:352–355.
Article13. Oshima H, Ishikawa T, Yoshida GJ, Naoi K, Maeda Y, Naka K, et al. TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene. 2014; 33:3820–3829.
Article14. Angelo LS, Kurzrock R. Vascular endothelial growth factor and its relationship to inflammatory mediators. Clin Cancer Res. 2007; 13:2825–2830.
Article15. Strowski MZ, Cramer T, Schäfer G, Jüttner S, Walduck A, Schipani E, et al. Helicobacter pylori stimulates host vascular endothelial growth factor-A (vegf-A) gene expression via MEK/ERK-dependent activation of Sp1 and Sp3. FASEB J. 2004; 18:218–220.
Article16. Karkkainen MJ, Petrova TV. Vascular endothelial growth factor receptors in the regulation of angiogenesis and lymphangiogenesis. Oncogene. 2000; 19:5598–5605.
Article17. Unemori EN, Ferrara N, Bauer EA, Amento EP. Vascular endothelial growth factor induces interstitial collagenase expression in human endothelial cells. J Cell Physiol. 1992; 153:557–562.
Article18. Wang X, Chen X, Fang J, Yang C. Overexpression of both VEGF-A and VEGF-C in gastric cancer correlates with prognosis, and silencing of both is effective to inhibit cancer growth. Int J Clin Exp Pathol. 2013; 6:586–597.19. Lee J, Gray A, Yuan J, Luoh SM, Avraham H, Wood WI. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc Natl Acad Sci U S A. 1996; 93:1988–1992.
Article20. Ishikawa M, Kitayama J, Kazama S, Nagawa H. Expression of vascular endothelial growth factor C and D (VEGF-C and -D) is an important risk factor for lymphatic metastasis in undifferentiated early gastric carcinoma. Jpn J Clin Oncol. 2003; 33:21–27.
Article21. Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Ylä-Herttuala S, Jäättelä M, et al. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001; 61:1786–1790.22. Neuchrist C, Erovic BM, Handisurya A, Fischer MB, Steiner GE, Hollemann D, et al. Vascular endothelial growth factor C and vascular endothelial growth factor receptor 3 expression in squamous cell carcinomas of the head and neck. Head Neck. 2003; 25:464–474.
Article23. Tsurusaki T, Kanda S, Sakai H, Kanetake H, Saito Y, Alitalo K, et al. Vascular endothelial growth factor-C expression in human prostatic carcinoma and its relationship to lymph node metastasis. Br J Cancer. 1999; 80:309–313.
Article24. Lee HS, Kim J. Constitutive expression of vascular endothelial cell growth factor (VEGF) gene family ligand and receptors on human upper and lower airway epithelial cells. Int Forum Allergy Rhinol. 2014; 4:8–14.
Article25. Kitadai Y, Amioka T, Haruma K, Tanaka S, Yoshihara M, Sumii K, et al. Clinicopathological significance of vascular endothelial growth factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J Cancer. 2001; 93:662–666.
Article26. Hashimoto I, Kodama J, Seki N, Hongo A, Yoshinouchi M, Okuda H, et al. Vascular endothelial growth factor-C expression and its relationship to pelvic lymph node status in invasive cervical cancer. Br J Cancer. 2001; 85:93–97.
Article27. Gabrilovich DI, Chen HL, Girgis KR, Cunningham HT, Meny GM, Nadaf S, et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996; 2:1096–1103.
Article28. Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997; 18:4–25.
Article29. Chu SC, Tsai CH, Yang SF, Huang FM, Su YF, Hsieh YS, et al. Induction of vascular endothelial growth factor gene expression by proinflammatory cytokines in human pulp and gingival fibroblasts. J Endod. 2004; 30:704–707.
Article30. Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat Med. 1996; 2:992–997.
Article31. Jones MK, Kawanaka H, Baatar D, Szabó IL, Tsugawa K, Pai R, et al. Gene therapy for gastric ulcers with single local injection of naked DNA encoding VEGF and angiopoietin-1. Gastroenterology. 2001; 121:1040–1047.
Article32. Brower V. Feeding the flame: new research adds to role of inflammation in cancer development. J Natl Cancer Inst. 2005; 97:251–253.
Article33. George ML, Tutton MG, Janssen F, Arnaout A, Abulafi AM, Eccles SA, et al. VEGF-A, VEGF-C, and VEGF-D in colorectal cancer progression. Neoplasia. 2001; 3:420–427.
Article34. Tian WY, Chen WC, Li R, Liu L. Markers CD40, VEGF, AKT, PI3K, and S100 correlate with tumor stage in gastric cancer. Onkologie. 2013; 36:26–31.
Article35. Tsugawa H, Suzuki H, Saya H, Hatakeyama M, Hirayama T, Hirata K, et al. Reactive oxygen species-induced autophagic degradation of Helicobacter pylori CagA is specifically suppressed in cancer stem-like cells. Cell Host Microbe. 2012; 12:764–777.
Article36. Ajami A, Shadman M, Rafiei A, Hosseini V, TalebiBezmin Abadi A, Alizadeh A, et al. Prevalence of EPIYA motifs in Helicobacter pylori strains isolated from patients with gastroduodenal disorders in northern Iran. Res Mol Med. 2013; 1:30–35.
Article37. Kondo K, Kaneko T, Baba M, Konno H. VEGF-C and VEGF-A synergistically enhance lymph node metastasis of gastric cancer. Biol Pharm Bull. 2007; 30:633–637.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Expression of Vascular Endothelial Growth Factor (VEGF)-C and VEGF-D in Early Gastric Cancer That's Invaded the Submucosa
- Vascular Endothelial Growth Factor and basic Fibroblast Growth Factor Expression in Early Gastric Carcinomas: Correlation with Clinicopathologic Factors
- Expression of Vascular Endothelial Growth Factors A,C and D in Gastric Adenocarcinoma
- Expression of Vascular Endothelial Growth Factor Correlated with Recurrence in Gastric Carcinomas
- Correlation of Vascular Endothelial Growth Factor-D Expression and VEGFR-3-Positive Vessel Density with Lymph Node Metastasis in Gastric Carcinoma