Korean J Physiol Pharmacol.
2023 Mar;27(2):187-196. 10.4196/kjpp.2023.27.2.187.
Negative self-regulation of transient receptor potential canonical 4 by the specific interaction with phospholipase C-δ1
- Affiliations
-
- 1Department of Physiology, Seoul National University College of Medicine, Seoul 03080, Korea
- KMID: 2539573
- DOI: http://doi.org/10.4196/kjpp.2023.27.2.187
Abstract
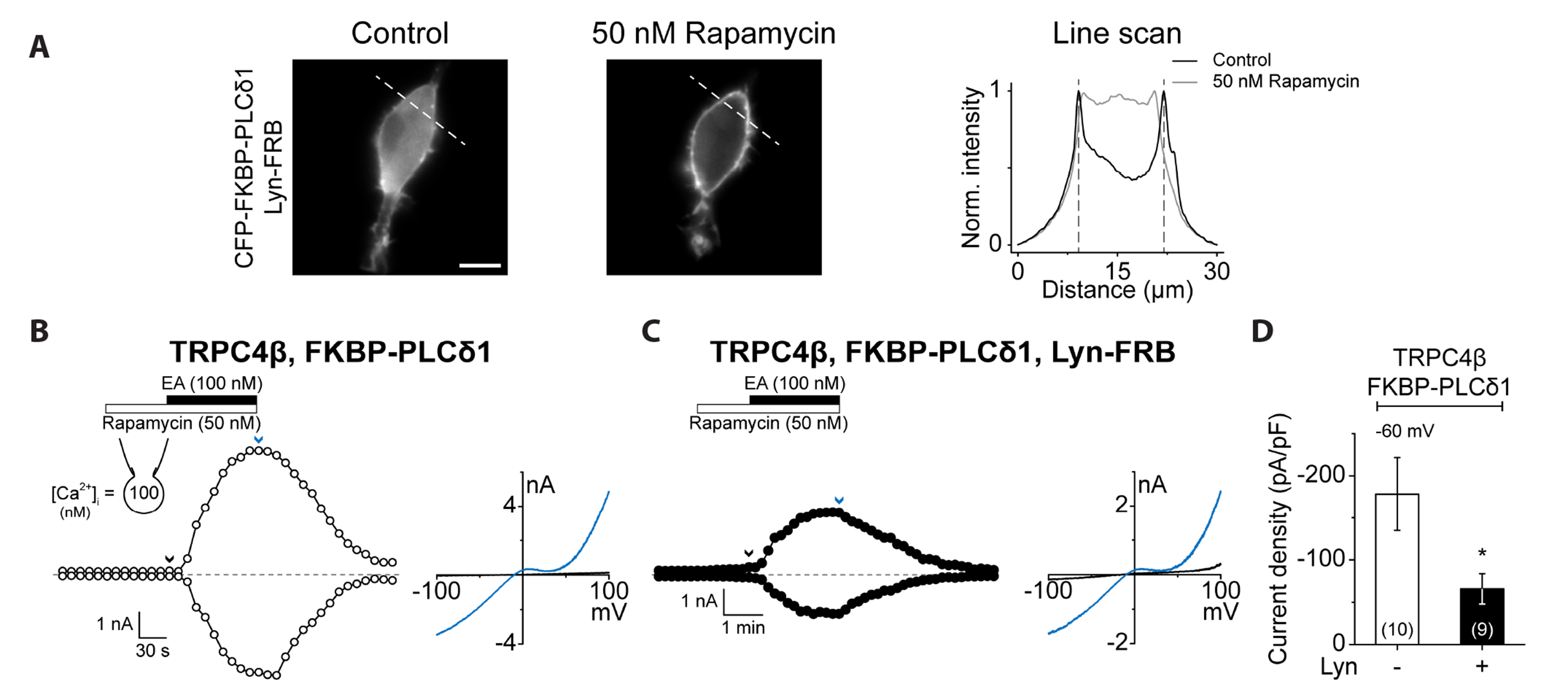
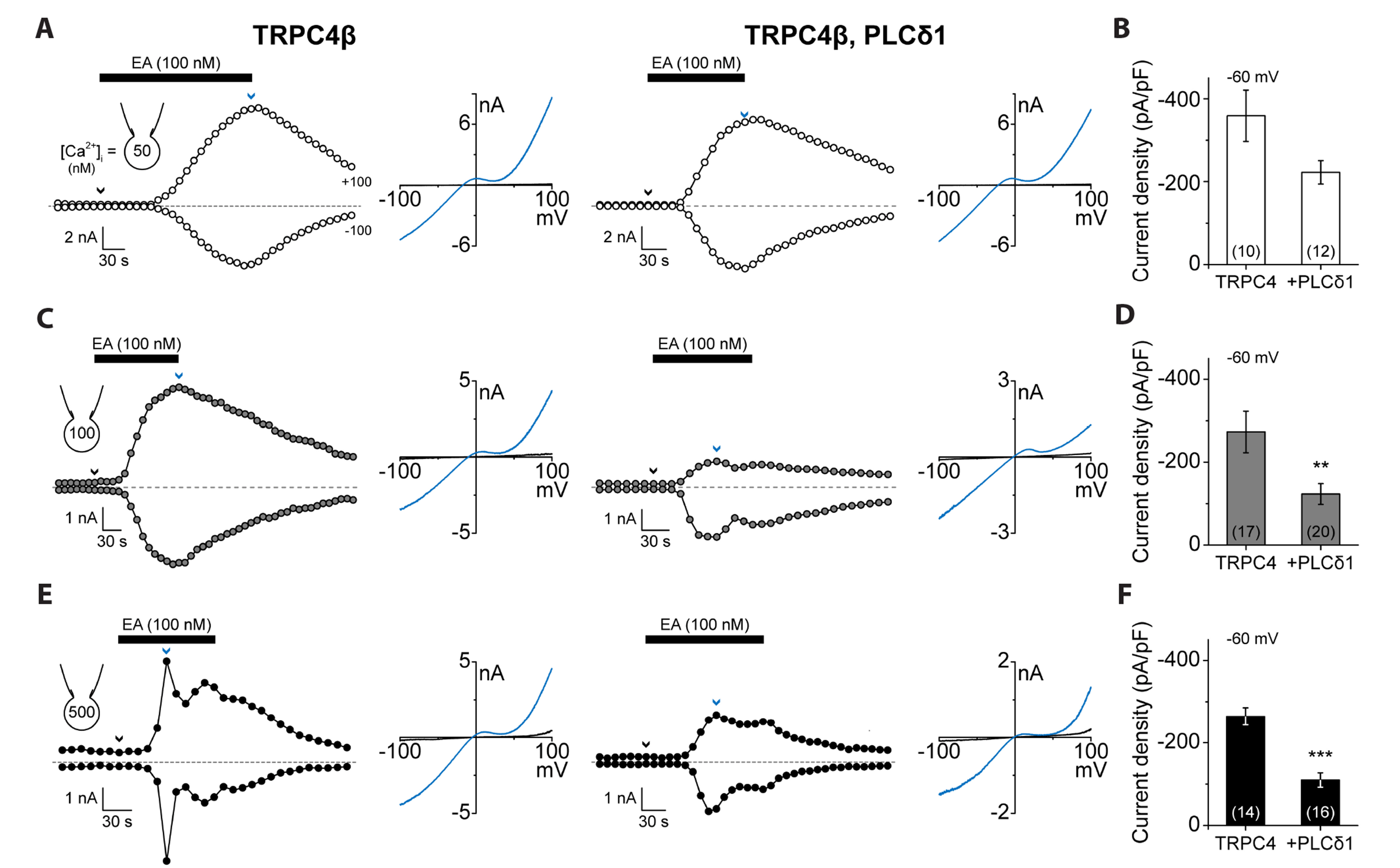
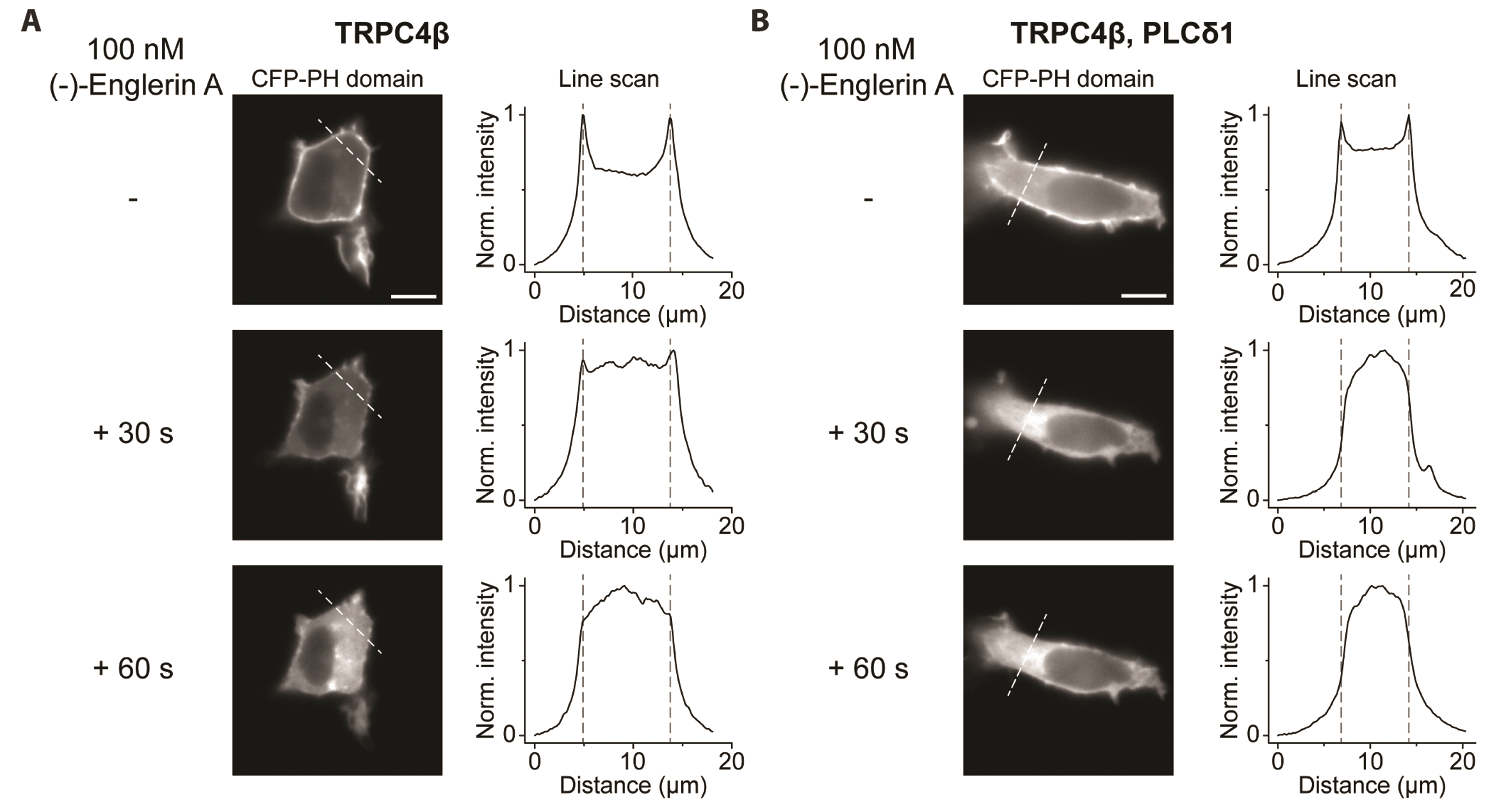
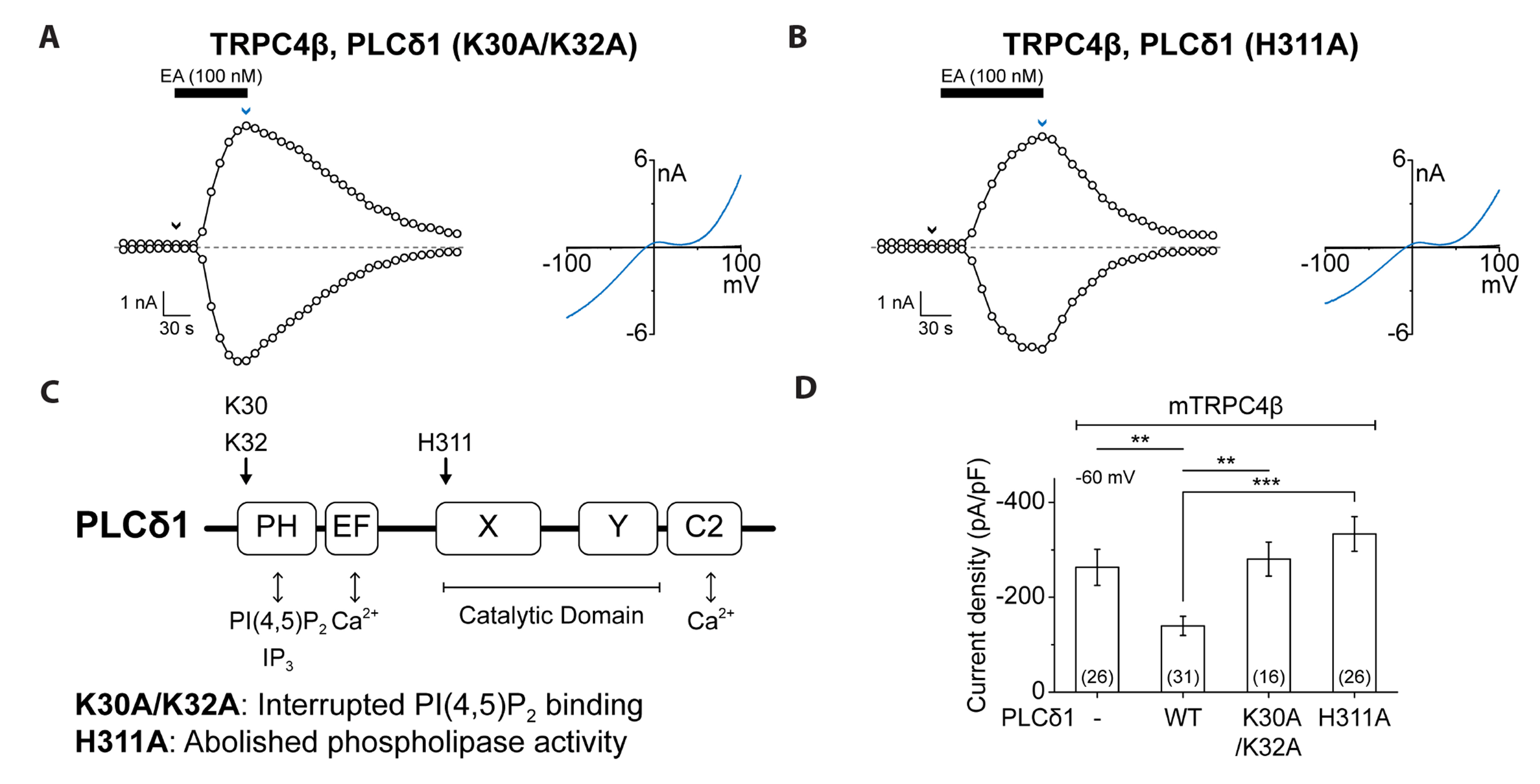
- Transient receptor potential canonical (TRPC) channels are non-selective calcium-permeable cation channels. It is suggested that TRPC4β is regulated by phospholipase C (PLC) signaling and is especially maintained by phosphatidylinositol 4,5-bisphosphate (PIP2 ). In this study, we present the regulation mechanism of the TRPC4 channel with PIP2 hydrolysis which is mediated by a channel-bound PLCδ1 but not by the GqPCR signaling pathway. Our electrophysiological recordings demonstrate that the Ca2+ via an open TRPC4 channel activates PLCδ1 in the physiological range, and it causes the decrease of current amplitude. The existence of PLCδ1 accelerated PIP2 depletion when the channel was activated by an agonist. Interestingly, PLCδ1 mutants which have lost the ability to regulate PIP2 level failed to reduce the TRPC4 current amplitude. Our results demonstrate that TRPC4 self-regulates its activity by allowing Ca2+ ions into the cell and promoting the PIP2 hydrolyzing activity of PLCδ1.
Keyword
Figure
Reference
-
1. Kim BJ, Kim MT, Jeon JH, Kim SJ, So I. 2008; Involvement of phosphatidylinositol 4,5-bisphosphate in the desensitization of canonical transient receptor potential 5. Biol Pharm Bull. 31:1733–1738. DOI: 10.1248/bpb.31.1733. PMID: 18758068. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=51449087751&origin=inward.
Article2. Kim H, Jeon JP, Hong C, Kim J, Myeong J, Jeon JH, So I. 2013; An essential role of PI(4,5)P₂ for maintaining the activity of the transient receptor potential canonical (TRPC)4β. Pflugers Arch. 465:1011–1021. DOI: 10.1007/s00424-013-1236-x. PMID: 23417604. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84879844737&origin=inward.
Article3. Ko J, Myeong J, Shin YC, So I. 2019; Differential PI(4,5)P₂ sensitivities of TRPC4, C5 homomeric and TRPC1/4, C1/5 heteromeric channels. Sci Rep. 9:1849. DOI: 10.1038/s41598-018-38443-0. PMID: 30755645. PMCID: PMC6372716. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85061476067&origin=inward.4. Otsuguro K, Tang J, Tang Y, Xiao R, Freichel M, Tsvilovskyy V, Ito S, Flockerzi V, Zhu MX, Zholos AV. 2008; Isoform-specific inhibition of TRPC4 channel by phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 283:10026–10036. DOI: 10.1074/jbc.M707306200. PMID: 18230622. PMCID: PMC2365920. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=44349107703&origin=inward.
Article5. Mederos Y Schnitzler M, Gudermann T, Storch U. 2018; Emerging roles of diacylglycerol-sensitive TRPC4/5 channels. Cells. 7:218. DOI: 10.3390/cells7110218. PMID: 30463370. PMCID: PMC6262340. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85071855162&origin=inward.
Article6. Storch U, Forst AL, Pardatscher F, Erdogmus S, Philipp M, Gregoritza M, Mederos Y Schnitzler M, Gudermann T. 2017; Dynamic NHERF interaction with TRPC4/5 proteins is required for channel gating by diacylglycerol. Proc Natl Acad Sci U S A. 114:E37–E46. DOI: 10.1073/pnas.1612263114. PMID: 27994151. PMCID: PMC5224389. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85008147871&origin=inward.
Article7. Zhu MH, Chae M, Kim HJ, Lee YM, Kim MJ, Jin NG, Yang DK, So I, Kim KW. 2005; Desensitization of canonical transient receptor potential channel 5 by protein kinase C. Am J Physiol Cell Physiol. 289:C591–C600. DOI: 10.1152/ajpcell.00440.2004. PMID: 15843439. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=23944515240&origin=inward.
Article8. Myeong J, Ko J, Kwak M, Kim J, Woo J, Ha K, Hong C, Yang D, Kim HJ, Jeon JH, So I. 2018; Dual action of the Gαq-PLCβ-PI(4,5)P₂ pathway on TRPC1/4 and TRPC1/5 heterotetramers. Sci Rep. 8:12117. DOI: 10.1038/s41598-018-30625-0. PMID: 30108272. PMCID: PMC6092394. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85053409035&origin=inward.
Article9. Ko J, Myeong J, Kwak M, Jeon JH, So I. 2019; Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels. Korean J Physiol Pharmacol. 23:357–366. DOI: 10.4196/kjpp.2019.23.5.357. PMID: 31496873. PMCID: PMC6717798. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85072645866&origin=inward.
Article10. Berridge MJ. 1993; Inositol trisphosphate and calcium signalling. Nature. 361:315–325. DOI: 10.1038/361315a0. PMID: 8381210. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0027397544&origin=inward.
Article11. Noh DY, Shin SH, Rhee SG. 1995; Phosphoinositide-specific phospholipase C and mitogenic signaling. Biochim Biophys Acta. 1242:99–113. DOI: 10.1016/0304-419X(95)00006-0. PMID: 7492569. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028971813&origin=inward.
Article12. Rhee SG, Choi KD. 1992; Regulation of inositol phospholipid-specific phospholipase C isozymes. J Biol Chem. 267:12393–12396. DOI: 10.1016/S0021-9258(18)42284-3. PMID: 1319994.
Article13. Lee SB, Rhee SG. 1995; Significance of PIP2 hydrolysis and regulation of phospholipase C isozymes. Curr Opin Cell Biol. 7:183–189. DOI: 10.1016/0955-0674(95)80026-3. PMID: 7612269. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028986998&origin=inward.
Article14. Kim YH, Park TJ, Lee YH, Baek KJ, Suh PG, Ryu SH, Kim KT. 1999; Phospholipase C-delta1 is activated by capacitative calcium entry that follows phospholipase C-beta activation upon bradykinin stimulation. J Biol Chem. 274:26127–26134. DOI: 10.1074/jbc.274.37.26127. PMID: 10473563. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033543237&origin=inward.
Article15. Allen V, Swigart P, Cheung R, Cockcroft S, Katan M. 1997; Regulation of inositol lipid-specific phospholipase cdelta by changes in Ca2+ ion concentrations. Biochem J. 327(Pt 2):545–552. DOI: 10.1042/bj3270545. PMID: 9359428. PMCID: PMC1218828. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0030681061&origin=inward.
Article16. Rebecchi MJ, Pentyala SN. 2000; Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 80:1291–1335. DOI: 10.1152/physrev.2000.80.4.1291. PMID: 11015615. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0033779101&origin=inward.
Article17. Cifuentes ME, Delaney T, Rebecchi MJ. 1994; D-myo-inositol 1,4,5-trisphosphate inhibits binding of phospholipase C-delta 1 to bilayer membranes. J Biol Chem. 269:1945–1948. DOI: 10.1016/S0021-9258(17)42118-1. PMID: 8294445.
Article18. Lukacs V, Thyagarajan B, Varnai P, Balla A, Balla T, Rohacs T. 2007; Dual regulation of TRPV1 by phosphoinositides. J Neurosci. 27:7070–7080. DOI: 10.1523/JNEUROSCI.1866-07.2007. PMID: 17596456. PMCID: PMC6672228. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=34347354387&origin=inward.
Article19. Rohács T, Lopes CM, Michailidis I, Logothetis DE. 2005; PI(4,5)P2 regulates the activation and desensitization of TRPM8 channels through the TRP domain. Nat Neurosci. 8:626–634. DOI: 10.1038/nn1451. PMID: 15852009. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=17844373771&origin=inward.
Article20. Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. 2001; Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 31:973–985. DOI: 10.1016/S0896-6273(01)00438-X. PMID: 11580897. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0035959942&origin=inward.
Article21. Epe B, Steinhäuser KG, Woolley P. 1983; Theory of measurement of Förster-type energy transfer in macromolecules. Proc Natl Acad Sci U S A. 80:2579–2583. DOI: 10.1073/pnas.80.9.2579. PMID: 16593305. PMCID: PMC393869.
Article22. Patterson G, Day RN, Piston D. 2001; Fluorescent protein spectra. J Cell Sci. 114(Pt 5):837–838. DOI: 10.1242/jcs.114.5.837. PMID: 11181166.
Article23. Kwak M, Hong C, Myeong J, Park EYJ, Jeon JH, So I. 2018; Gαi-mediated TRPC4 activation by polycystin-1 contributes to endothelial function via STAT1 activation. Sci Rep. 8:3480. DOI: 10.1038/s41598-018-21873-1. PMID: 29472562. PMCID: PMC5823873. PMID: 6b1dac94af454f40b779ad80c02bde32. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=85042546595&origin=inward.24. Blair NT, Kaczmarek JS, Clapham DE. 2009; Intracellular calcium strongly potentiates agonist-activated TRPC5 channels. J Gen Physiol. 133:525–546. DOI: 10.1085/jgp.200810153. PMID: 19398778. PMCID: PMC2712973. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=66049141826&origin=inward.
Article25. Garcia P, Gupta R, Shah S, Morris AJ, Rudge SA, Scarlata S, Petrova V, McLaughlin S, Rebecchi MJ. 1995; The pleckstrin homology domain of phospholipase C-delta 1 binds with high affinity to phosphatidylinositol 4,5-bisphosphate in bilayer membranes. Biochemistry. 34:16228–16234. DOI: 10.1021/bi00049a039. PMID: 8519781. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028787055&origin=inward.
Article26. Ellis MV, U S, Katan M. 1995; Mutations within a highly conserved sequence present in the X region of phosphoinositide-specific phospholipase C-delta 1. Biochem J. 307(Pt 1):69–75. DOI: 10.1042/bj3070069. PMID: 7717996. PMCID: PMC1136746. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0028937655&origin=inward.
Article27. Zhang Z, Okawa H, Wang Y, Liman ER. 2005; Phosphatidylinositol 4,5-bisphosphate rescues TRPM4 channels from desensitization. J Biol Chem. 280:39185–39192. DOI: 10.1074/jbc.M506965200. PMID: 16186107. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=28244499948&origin=inward.
Article28. Liu D, Liman ER. 2003; Intracellular Ca2+ and the phospholipid PIP2 regulate the taste transduction ion channel TRPM5. Proc Natl Acad Sci U S A. 100:15160–15165. DOI: 10.1073/pnas.2334159100. PMID: 14657398. PMCID: PMC299934. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0344149569&origin=inward.
Article29. Liu B, Qin F. 2005; Functional control of cold- and menthol-sensitive TRPM8 ion channels by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 25:1674–1681. DOI: 10.1523/JNEUROSCI.3632-04.2005. PMID: 15716403. PMCID: PMC6725927. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=14044261739&origin=inward.
Article30. Daniels RL, Takashima Y, McKemy DD. 2009; Activity of the neuronal cold sensor TRPM8 is regulated by phospholipase C via the phospholipid phosphoinositol 4,5-bisphosphate. J Biol Chem. 284:1570–1582. DOI: 10.1074/jbc.M807270200. PMID: 19019830. PMCID: PMC2615505. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=59449084852&origin=inward.
Article31. Yudin Y, Lutz B, Tao YX, Rohacs T. 2016; Phospholipase C δ4 regulates cold sensitivity in mice. J Physiol. 594:3609–3628. DOI: 10.1113/JP272321. PMID: 27062607. PMCID: PMC4929334. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84977591935&origin=inward.
Article32. Lukacs V, Yudin Y, Hammond GR, Sharma E, Fukami K, Rohacs T. 2013; Distinctive changes in plasma membrane phosphoinositides underlie differential regulation of TRPV1 in nociceptive neurons. J Neurosci. 33:11451–11463. DOI: 10.1523/JNEUROSCI.5637-12.2013. PMID: 23843517. PMCID: PMC3724548. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84880435653&origin=inward.
Article33. Banno Y, Okano Y, Nozawa Y. 1994; Thrombin-mediated phosphoinositide hydrolysis in Chinese hamster ovary cells overexpressing phospholipase C-delta 1. J Biol Chem. 269:15846–15852. DOI: 10.1016/S0021-9258(17)40758-7. PMID: 8195239.
Article34. Tsvilovskyy VV, Zholos AV, Aberle T, Philipp SE, Dietrich A, Zhu MX, Birnbaumer L, Freichel M, Flockerzi V. 2009; Deletion of TRPC4 and TRPC6 in mice impairs smooth muscle contraction and intestinal motility in vivo. Gastroenterology. 137:1415–1424. DOI: 10.1053/j.gastro.2009.06.046. PMID: 19549525. PMCID: PMC2757464. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=70349432267&origin=inward.
Article35. Dresviannikov AV, Bolton TB, Zholos AV. 2006; Muscarinic receptor-activated cationic channels in murine ileal myocytes. Br J Pharmacol. 149:179–187. DOI: 10.1038/sj.bjp.0706852. PMID: 16894345. PMCID: PMC2013797. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33748480349&origin=inward.
Article36. Lee KP, Jun JY, Chang IY, Suh SH, So I, Kim KW. 2005; TRPC4 is an essential component of the nonselective cation channel activated by muscarinic stimulation in mouse visceral smooth muscle cells. Mol Cells. 20:435–441. PMID: 16404161. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=33644869882&origin=inward.37. Thakur DP, Tian JB, Jeon J, Xiong J, Huang Y, Flockerzi V, Zhu MX. 2016; Critical roles of Gi/o proteins and phospholipase C-δ1 in the activation of receptor-operated TRPC4 channels. Proc Natl Acad Sci U S A. 113:1092–1097. DOI: 10.1073/pnas.1522294113. PMID: 26755577. PMCID: PMC4743816. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=84955450086&origin=inward.
Article38. Klein RM, Ufret-Vincenty CA, Hua L, Gordon SE. 2008; Determinants of molecular specificity in phosphoinositide regulation. Phosphatidylinositol (4,5)-bisphosphate (PI(4,5)P2) is the endogenous lipid regulating TRPV1. J Biol Chem. 283:26208–26216. DOI: 10.1074/jbc.M801912200. PMID: 18574245. PMCID: PMC2533779. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=54449099889&origin=inward.39. Liu B, Zhang C, Qin F. 2005; Functional recovery from desensitization of vanilloid receptor TRPV1 requires resynthesis of phosphatidylinositol 4,5-bisphosphate. J Neurosci. 25:4835–4843. DOI: 10.1523/JNEUROSCI.1296-05.2005. PMID: 15888659. PMCID: PMC6724779. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=18644381102&origin=inward.
Article40. Rohacs T, Thyagarajan B, Lukacs V. 2008; Phospholipase C mediated modulation of TRPV1 channels. Mol Neurobiol. 37:153–163. DOI: 10.1007/s12035-008-8027-y. PMID: 18528787. PMCID: PMC2568872. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=53749084409&origin=inward.
Article41. Murthy SN, Lomasney JW, Mak EC, Lorand L. 1999; Interactions of G(h)/transglutaminase with phospholipase Cdelta1 and with GTP. Proc Natl Acad Sci U S A. 96:11815–11819. DOI: 10.1073/pnas.96.21.11815. PMID: 10518533. PMCID: PMC18369. PMID: https://www.scopus.com/inward/record.uri?partnerID=HzOxMe3b&scp=0032718477&origin=inward.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of phospholipase C β downstream effect on transient receptor potential canonical 1/4, transient receptor potential canonical 1/5 channels
- Immunohistochemical Study on the Distribution of Canonical Transient Receptor Potential Channels in Rat Cerebellum
- Analysis of interaction between intracellular spermine and transient receptor potential canonical 4 channel: multiple candidate sites of negatively charged amino acids for the inward rectification of transient receptor potential canonical 4
- Canonical Transient Receptor Potential Channels and Their Link with Cardio/Cerebro-Vascular Diseases
- The mechanism of phospholipase C-gamma1 regulation