J Pathol Transl Med.
2017 Jan;51(1):9-16. 10.4132/jptm.2016.08.23.
Increased Expression of Thymosin β₄ Is Independently Correlated with Hypoxia Inducible Factor-1α (HIF-1α) and Worse Clinical Outcome in Human Colorectal Cancer
- Affiliations
-
- 1Department of Pathology, Eulji University Hospital, Daejeon, Korea. kjh2000@eulji.ac.kr astrias@eulji.ac.kr
- 2Department of Surgery, Eulji University Hospital, Daejeon, Korea.
- KMID: 2367675
- DOI: http://doi.org/10.4132/jptm.2016.08.23
Abstract
- BACKGROUND
Thymosin β₄ is a multi-functional hormone-like polypeptide, being involved in cell migration, angiogenesis, and tumor metastasis. This study was undertaken to clarify the clinicopathologic implications of thymosin β₄ expression in human colorectal cancers (CRCs).
METHODS
We investigated tissue sections from 143 patients with CRC by immunohistochemistry. In addition, we evaluated the expression patterns and the clinico-pathological significance of thymosin β₄ expression in association with hypoxia inducible factor-1α (HIF-1α) expression in the CRC series.
RESULTS
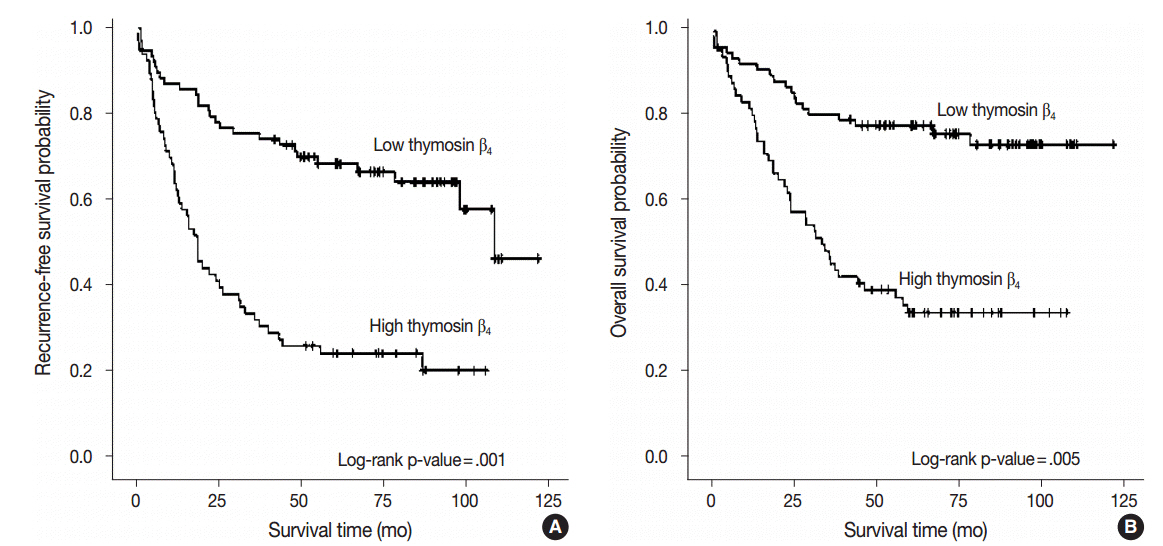
High expression of thymosin β₄ was significantly correlated with lymphovascular invasion, invasion depth, regional lymph node metastasis, distant metastasis, and TNM stage. Patients with high expression of thymosin β₄ showed poor recurrence-free survival (p = .001) and poor overall survival (p = .005) on multivariate analysis. We also found that thymosin β4 and HIF-1α were overexpressed and that thymosin β₄ expression increased in parallel with HIF-1α expression in CRC.
CONCLUSIONS
A high expression level of thymosin β₄ indicates poor clinical outcomes and may be a useful prognostic factor in CRC. Thymosin β₄ is functionally related with HIF-1α and may be a potentially valuable biomarker and possible therapeutic target for CRC.
Keyword
MeSH Terms
Figure
Reference
-
1. Cannito S, Novo E, Compagnone A, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008; 29:2267–78.
Article2. Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-κB via c-SRC and oxidant-dependent cell death. Cancer Res. 2007; 67:7368–77.
Article3. Sansone P, Piazzi G, Paterini P, et al. Cyclooxygenase-2/carbonic anhydrase-IX up-regulation promotes invasive potential and hypoxia survival in colorectal cancer cells. J Cell Mol Med. 2009; 13:3876–87.
Article4. To KK, Koshiji M, Hammer S, Huang LE. Genetic instability: the dark side of the hypoxic response. Cell Cycle. 2005; 4:881–2.
Article5. Huang LE, Bindra RS, Glazer PM, Harris AL. Hypoxia-induced genetic instability: a calculated mechanism underlying tumor progression. J Mol Med (Berl). 2007; 85:139–48.6. Bristow RG, Hill RP. Hypoxia and metabolism: hypoxia, DNA repair and genetic instability. Nat Rev Cancer. 2008; 8:180–92.7. Oh JM, Ryoo IJ, Yang Y, Kim HS, Yang KH, Moon EY. Hypoxia-inducible transcription factor (HIF)-1 alpha stabilization by actin-sequestering protein, thymosin beta-4 (TB4) in Hela cervical tumor cells. Cancer Lett. 2008; 264:29–35.8. Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005; 22:25–30.
Article9. Sivridis E, Giatromanolaki A, Koukourakis MI. Proliferating fibroblasts at the invading tumour edge of colorectal adenocarcinomas are associated with endogenous markers of hypoxia, acidity, and oxidative stress. J Clin Pathol. 2005; 58:1033–8.
Article10. Giles RH, Lolkema MP, Snijckers CM, et al. Interplay between VHL/HIF1alpha and Wnt/beta-catenin pathways during colorectal tumorigenesis. Oncogene. 2006; 25:3065–70.11. Koukourakis MI, Giatromanolaki A, Polychronidis A, et al. Endogenous markers of hypoxia/anaerobic metabolism and anemia in primary colorectal cancer. Cancer Sci. 2006; 97:582–8.
Article12. Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. Beta-thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001; 33:205–20.13. Kobayashi T, Okada F, Fujii N, et al. Thymosin-beta4 regulates motility and metastasis of malignant mouse fibrosarcoma cells. Am J Pathol. 2002; 160:869–82.14. Clark EA, Golub TR, Lander ES, Hynes RO. Genomic analysis of metastasis reveals an essential role for RhoC. Nature. 2000; 406:532–5.
Article15. Xie D, Jauch A, Miller CW, Bartram CR, Koeffler HP. Discovery of over-expressed genes and genetic alterations in breast cancer cells using a combination of suppression subtractive hybridization, multiplex FISH and comparative genomic hybridization. Int J Oncol. 2002; 21:499–507.
Article16. Kim L, Kim YJ, Choi SJ, et al. Prognostic significance of thymosin- 4 in gastric adenocarcinoma patients. Korean J Pathol. 2007; 41:176–82.17. Hsiao HL, Wang WS, Chen PM, Su Y. Overexpression of thymosin beta-4 renders SW480 colon carcinoma cells more resistant to apoptosis triggered by FasL and two topoisomerase II inhibitors via downregulating Fas and upregulating Survivin expression, respectively. Carcinogenesis. 2006; 27:936–44.18. Huang HC, Hu CH, Tang MC, Wang WS, Chen PM, Su Y. Thymosin beta4 triggers an epithelial-mesenchymal transition in colorectal carcinoma by upregulating integrin-linked kinase. Oncogene. 2007; 26:2781–90.19. Wang WS, Chen PM, Hsiao HL, Ju SY, Su Y. Overexpression of the thymosin beta-4 gene is associated with malignant progression of SW480 colon cancer cells. Oncogene. 2003; 22:3297–306.20. Wang WS, Chen PM, Hsiao HL, Wang HS, Liang WY, Su Y. Overexpression of the thymosin beta-4 gene is associated with increased invasion of SW480 colon carcinoma cells and the distant metastasis of human colorectal carcinoma. Oncogene. 2004; 23:6666–71.21. Wang WS, Chen PM, Su Y. Colorectal carcinoma: from tumorigenesis to treatment. Cell Mol Life Sci. 2006; 63:663–71.
Article22. Piao Z, Hong CS, Jung MR, Choi C, Park YK. Thymosin beta4 induces invasion and migration of human colorectal cancer cells through the ILK/AKT/beta-catenin signaling pathway. Biochem Biophys Res Commun. 2014; 452:858–64.23. Kang YJ, Jo JO, Ock MS, et al. Thymosin beta4 was upregulated in recurred colorectal cancers. J Clin Pathol. 2014; 67:188–90.24. Yoon SY, Lee HR, Park Y, et al. Thymosin beta4 expression correlates with lymph node metastasis through hypoxia inducible factor-alpha induction in breast cancer. Oncol Rep. 2011; 25:23–31.
Article25. Washington MK, Berlin J, Branton P, et al. Protocol for the examination of specimens from patients with primary carcinoma of the colon and rectum. Arch Pathol Lab Med. 2009; 133:1539–51.
Article26. Prall F. Tumour budding in colorectal carcinoma. Histopathology. 2007; 50:151–62.
Article27. Wang LM, Kevans D, Mulcahy H, et al. Tumor budding is a strong and reproducible prognostic marker in T3N0 colorectal cancer. Am J Surg Pathol. 2009; 33:134–41.
Article28. Puppa G. TNM staging system of colorectal carcinoma: surgical pathology of the seventh edition. Diagn Histopathol. 2011; 17:243–62.
Article29. Höckel M, Vaupel P. Biological consequences of tumor hypoxia. Semin Oncol. 2001; 28(2 Suppl 8):36–41.30. Foo SS, Abbott DF, Lawrentschuk N, Scott AM. Functional imaging of intratumoral hypoxia. Mol Imaging Biol. 2004; 6:291–305.
Article31. Mabjeesh NJ, Amir S. Hypoxia-inducible factor (HIF) in human tumorigenesis. Histol Histopathol. 2007; 22:559–72.32. Lee JW, Ryu YK, Ji YH, Kang JH, Moon EY. Hypoxia/reoxygenation-experienced cancer cell migration and metastasis are regulated by Rap1- and Rac1-GTPase activation via the expression of thymosin beta-4. Oncotarget. 2015; 6:9820–33.
Article33. Tang MC, Chan LC, Yeh YC, et al. Thymosin beta 4 induces colon cancer cell migration and clinical metastasis via enhancing ILK/IQGAP1/Rac1 signal transduction pathway. Cancer Lett. 2011; 308:162–71.
Article34. Nemolato S, Restivo A, Cabras T, et al. Thymosin beta 4 in colorectal cancer is localized predominantly at the invasion front in tumor cells undergoing epithelial mesenchymal transition. Cancer Biol Ther. 2012; 13:191–7.35. Jo JO, Kim SR, Bae MK, et al. Thymosin beta4 induces the expression of vascular endothelial growth factor (VEGF) in a hypoxia-inducible factor (HIF)-1alpha-dependent manner. Biochim Biophys Acta. 2010; 1803:1244–51.36. Brahimi-Horn MC, Pouysségur J. HIF at a glance. J Cell Sci. 2009; 122(Pt 8):1055–7.
Article37. Ryan HE, Poloni M, McNulty W, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000; 60:4010–5.38. Swami M. Hypoxia: the HIF2alpha puzzle. Nat Rev Cancer. 2010; 10:603.39. Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998; 7:205–13.40. Kietzmann T, Cornesse Y, Brechtel K, Modaressi S, Jungermann K. Perivenous expression of the mRNA of the three hypoxia-inducible factor alpha-subunits, HIF1alpha, HIF2alpha and HIF3alpha, in rat liver. Biochem J. 2001; 354(Pt 3):531–7.41. Clottes E. Hypoxia-inducible factor 1: regulation, involvement in carcinogenesis and target for anticancer therapy. Bull Cancer. 2005; 92:119–27.42. Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in common human cancers and their metastases. Cancer Res. 1999; 59:5830–5.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia-Inducible Factor 1α Regulates the Transforming Growth Factor β1/SMAD Family Member 3 Pathway to Promote Breast Cancer Progression
- Hypoxia Inducible Factor-1α Directly Induces the Expression of Receptor Activator of Nuclear Factor-κB Ligand in Chondrocytes
- Effects of Histone Deacetylase Inhibitor (Valproic Acid) on the Expression of Hypoxia-inducible Factor-1 Alpha in Human Retinal Müller Cells
- Genipin Inhibits Hypoxia-Induced Accumulation of HIF-1α and VEGF Expressions in Human Cervical Carcinoma Cells
- Overexpression of CD44 Standard Isoform Upregulates HIF-1α Signaling in Hypoxic Breast Cancer Cells