J Breast Cancer.
2018 Sep;21(3):259-266. 10.4048/jbc.2018.21.e42.
Hypoxia-Inducible Factor 1α Regulates the Transforming Growth Factor β1/SMAD Family Member 3 Pathway to Promote Breast Cancer Progression
- Affiliations
-
- 1Department of Physical Examination, The First Affiliated Hospital of Chongqing Medical University, Chongqing, China. liang-ran@outlook.com
- 2Department of Breast and Thyroid Surgery, Renmin Hospital of Wuhan University, Wuhan, China.
- KMID: 2421366
- DOI: http://doi.org/10.4048/jbc.2018.21.e42
Abstract
- PURPOSE
The transforming growth factor β1 (TGF-β1)/SMAD family member 3 (SMAD3) pathway, and hypoxia-inducible factor 1α (HIF-1α) are two key players in various types of malignancies including breast cancer. The TGF-β1/SMAD3 pathway can interact with HIF-1α in some diseases; however, their interaction in breast cancer is still unknown. Therefore, our study aimed to investigate the interactions between the TGF-β1/SMAD3 pathway and HIF-1α in breast cancer.
METHODS
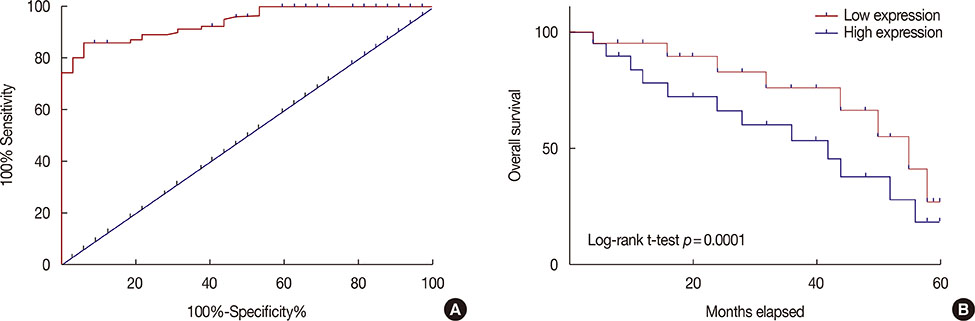
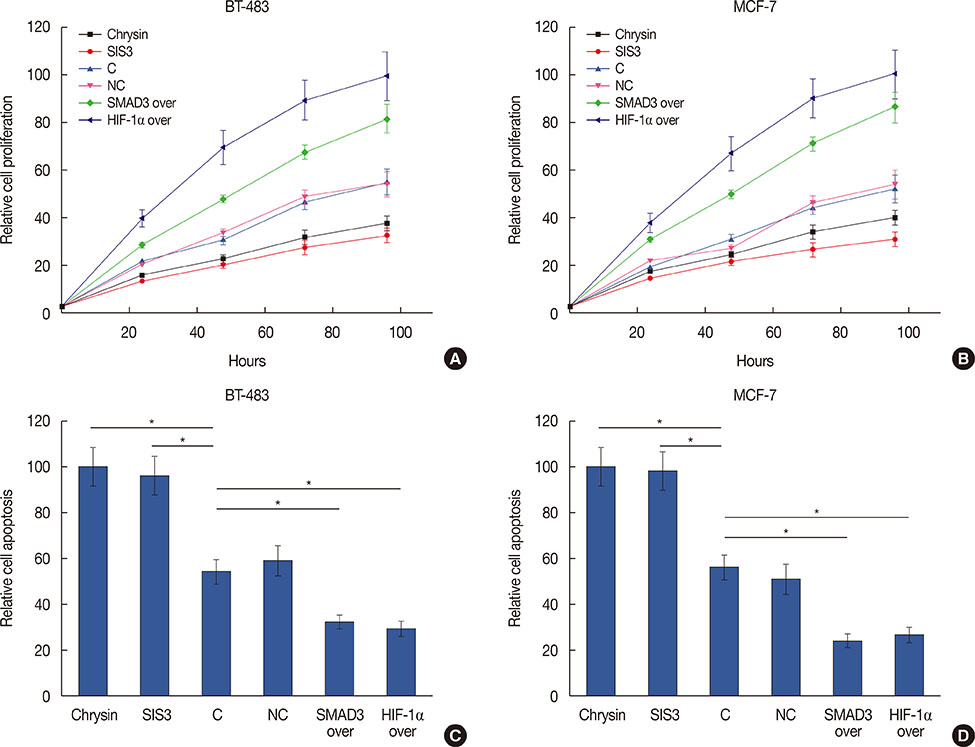
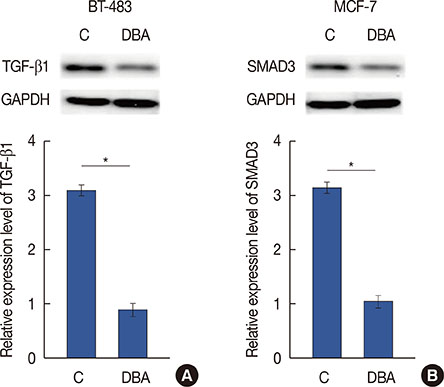
Expression of HIF-1α in serum of breast cancer patients and healthy controls was detected by quantitative reverse transcription polymerase chain reaction, and the diagnostic value of HIF-1α for breast cancer was evaluated by receiver operating characteristic curve analysis. Breast cancer cell lines overexpressing SMAD3 and HIF-1α were established. Cell apoptosis and proliferation following different treatments were detected by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, and cell counting kit-8, respectively. Expression of related proteins was detected by western blot.
RESULTS
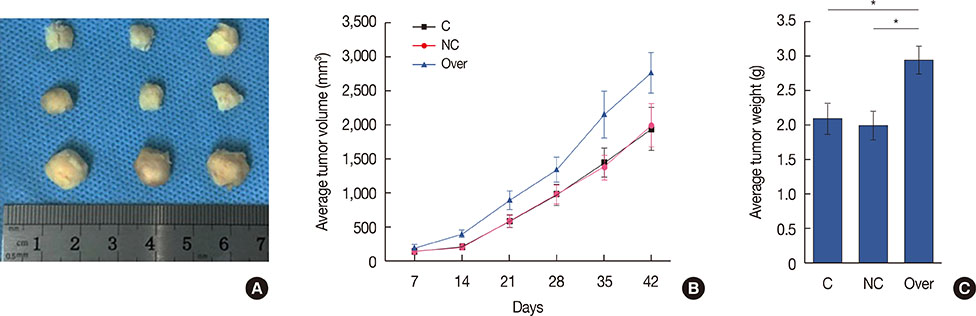
Serum levels of HIF-1α were higher in breast cancer patients than in normal controls. Both SMAD3 and HIF-1α overexpression inhibited cell apoptosis and promoted cell proliferation. Treatment with inhibitors of HIF-1α and SMAD3 promoted apoptosis in breast cancer cells and inhibited their proliferation. Overexpression of HIF-1α promoted the expression of TGF-β1 and SMAD3, while SMAD3 overexpression did not significantly affect expression of HIF-1α or TGF-β1.
CONCLUSION
HIF-1α serves as an upstream regulator of the TGF-β1/SMAD3 pathway and promotes the growth of breast cancer.
Keyword
MeSH Terms
-
Apoptosis
Blotting, Western
Breast Neoplasms*
Breast*
Cell Count
Cell Line
Cell Proliferation
Humans
Hypoxia-Inducible Factor 1
Polymerase Chain Reaction
Reverse Transcription
ROC Curve
Smad3 Protein
Transforming Growth Factor beta1
Transforming Growth Factors*
Hypoxia-Inducible Factor 1
Smad3 Protein
Transforming Growth Factor beta1
Transforming Growth Factors
Figure
Reference
-
1. DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017; 67:439–448.
Article2. Ward EM, DeSantis CE, Lin CC, Kramer JL, Jemal A, Kohler B, et al. Cancer statistics: breast cancer in situ. CA Cancer J Clin. 2015; 65:481–495.
Article3. Daly B, Olopade OI. A perfect storm: how tumor biology, genomics, and health care delivery patterns collide to create a racial survival disparity in breast cancer and proposed interventions for change. CA Cancer J Clin. 2015; 65:221–238.
Article4. Carroll JS, Hickey TE, Tarulli GA, Williams M, Tilley WD. Deciphering the divergent roles of progestogens in breast cancer. Nat Rev Cancer. 2017; 17:54–64.
Article5. O'Driscoll L, Clynes M. Biomarkers and multiple drug resistance in breast cancer. Curr Cancer Drug Targets. 2006; 6:365–384.6. Ellis LM, Hicklin DJ. Resistance to targeted therapies: refining anticancer therapy in the era of molecular oncology. Clin Cancer Res. 2009; 15:7471–7478.
Article7. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; 3:721–732.
Article8. Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, et al. Fatty acid uptake and lipid storage induced by HIF-1alpha contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014; 9:349–365.
Article9. Blancher C, Moore JW, Talks KL, Houlbrook S, Harris AL. Relationship of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha expression to vascular endothelial growth factor induction and hypoxia survival in human breast cancer cell lines. Cancer Res. 2000; 60:7106–7113.10. Xiang L, Gilkes DM, Hu H, Luo W, Bullen JW, Liang H, et al. HIF-1alpha and TAZ serve as reciprocal co-activators in human breast cancer cells. Oncotarget. 2015; 6:11768–11778.
Article11. Wang J, Ni Z, Duan Z, Wang G, Li F. Altered expression of hypoxia-inducible factor-1alpha (HIF-1alpha) and its regulatory genes in gastric cancer tissues. PLoS One. 2014; 9:e99835.12. Cho KH, Choi MJ, Jeong KJ, Kim JJ, Hwang MH, Shin SC, et al. A ROS/STAT3/HIF-1 alpha signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate. 2014; 74:528–536.
Article13. Nalwoga H, Ahmed L, Arnes JB, Wabinga H, Akslen LA. Strong expression of hypoxia-inducible factor-1alpha (HIF-1alpha) is associated with axl expression and features of aggressive tumors in African breast cancer. PLoS One. 2016; 11:e0146823.14. Li G, Shan C, Liu L, Zhou T, Zhou J, Hu X, et al. Tanshinone IIA inhibits HIF-1alpha and VEGF expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1 signaling pathway. PLoS One. 2015; 10:e0117440.15. Pipinikas CP, Carter ND, Corbishley CM, Fenske CD. HIF-1alpha mRNA gene expression levels in improved diagnosis of early stages of prostate cancer. Biomarkers. 2008; 13:680–691.
Article16. Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Oberhuber G. Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res. 2000; 60:4693–4696.17. Kim SJ, Rabbani ZN, Dewhirst MW, Vujaskovic Z, Vollmer RT, Schreiber EG, et al. Expression of HIF-1alpha, CA IX, VEGF, and MMP-9 in surgically resected non-small cell lung cancer. Lung Cancer. 2005; 49:325–335.
Article18. Jia ZZ, Jiang GM, Feng YL. Serum HIF-1alpha and VEGF levels pre- and post-TACE in patients with primary liver cancer. Chin Med Sci J. 2011; 26:158–162.
Article19. He G, Jiang Y, Zhang B, Wu G. The effect of HIF-1alpha on glucose metabolism, growth and apoptosis of pancreatic cancerous cells. Asia Pac J Clin Nutr. 2014; 23:174–180.20. Chen HS, Bai MH, Zhang T, Li GD, Liu M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-beta/Smad3 signaling pathway in human breast cancer MCF-7 cells. Int J Oncol. 2015; 46:1730–1738.
Article21. Kushida N, Nomura S, Mimura I, Fujita T, Yamamoto S, Nangaku M, et al. Hypoxia-inducible factor-1alpha activates the transforming growth factor-beta/smad3 pathway in kidney tubular epithelial cells. Am J Nephrol. 2016; 44:276–285.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Tripartite Motif Family Proteins in TGF-β Signaling Pathway and Cancer
- Role of the transforming growth factor (TGF)-β1 and TGF-β1 signaling pathway on the pathophysiology of respiratory pneumococcal infections
- Overexpression of CD44 Standard Isoform Upregulates HIF-1α Signaling in Hypoxic Breast Cancer Cells
- Clinicopathological Significance of SMAD4 Expression in Breast Cancer
- Pine bark extract (Pycnogenol®) suppresses cigarette smoke-induced fibrotic response via transforming growth factor-β1/Smad family member 2/3 signaling