J Bacteriol Virol.
2016 Dec;46(4):288-294. 10.4167/jbv.2016.46.4.288.
Acrosorium polyneurum Extract Inhibits the LPS-Induced Inflammatory Response by Impairing the MAPK and NF-κB Pathways
- Affiliations
-
- 1Department of Microbiology and Immunology, School of Medicine and Brain Korea 21 PLUS Program, Jeju National University, Jeju, Korea. yskoh7@jejunu.ac.kr
- 2Institute of Medical Science, Jeju National University, Jeju, Korea.
- KMID: 2366875
- DOI: http://doi.org/10.4167/jbv.2016.46.4.288
Abstract
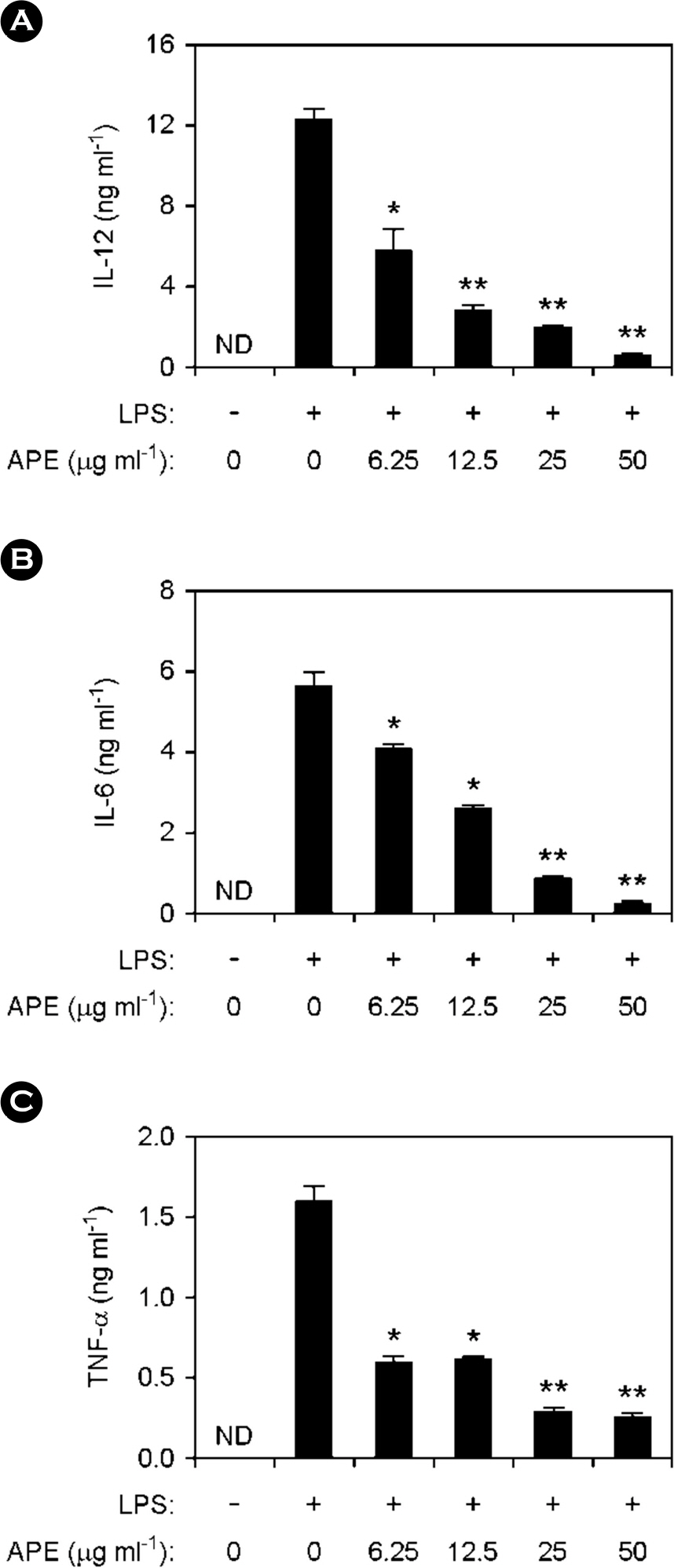
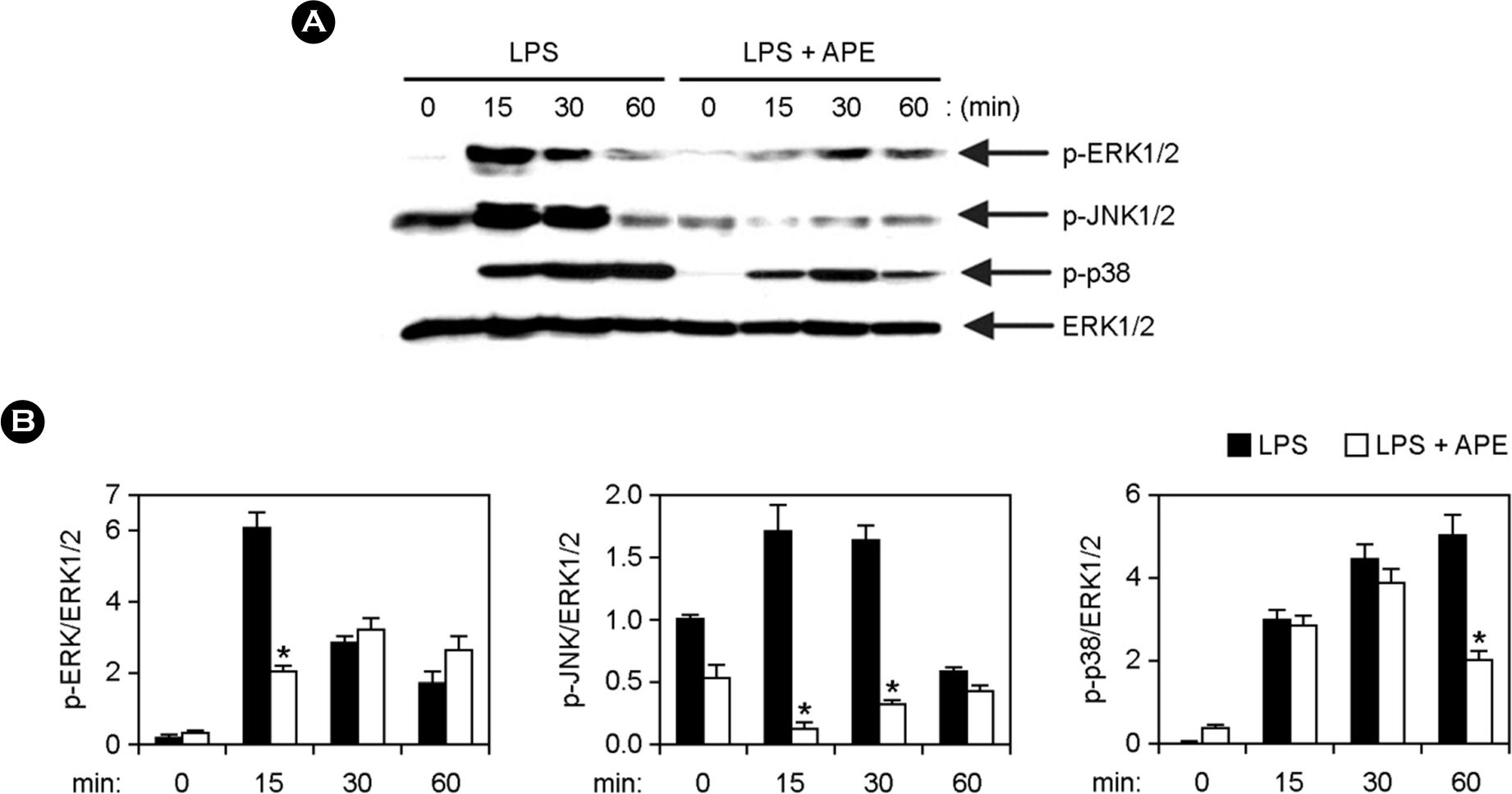
- Marine algae exhibit broad spectrum anti-bacterial and anti-inflammatory activities. Acrosorium polyneurum (A. polyneurum) is a marine red alga and belongs to the family Delesseriaceae. The present research evaluates the antiinflammatory effects of A. polyneurum extract (APE) on pro-inflammatory cytokine production. APE demonstrated substantial inhibitory effects on production of pro-inflammatory cytokine in bone marrow-derived macrophages (BMDMs). APE pre-treatment in the lipopolysaccharide (LPS)-stimulated BMDMs exhibited a robust inhibitory effect on production of interleukin (IL)-12, IL-6 and tumor necrosis factor (TNF)-α. It revealed a robust inhibitory effect on phosphorylation of ERK1/2, JNK1/2 and p38. APE also showed remarkable inhibitory effect on phosphorylation and degradation of IκBα. Furthermore, APE pre-treatment demonstrated substantial inhibition of LPS-induced production of nitric oxide and inducible nitric oxide synthase. Collectively, these data suggest that APE has a noteworthy anti-inflammatory property and deserve further studies concerning its potential use as a medicinal agent for inflammation-related disorders.
Keyword
MeSH Terms
Figure
Reference
-
1). Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010; 140:805–20.
Article2). Manzoor Z, Koh YS. Mitogen-activated protein kinases in inflammation. J Bacteriol Virol. 2012; 42:189–95.
Article3). Koh YS. Nucleic acid recognition and signaling by Toll-like receptor 9: Compartment-dependent regulation. J Bacteriol Virol. 2011; 41:131–2.
Article4). Efron PA, Tsujimoto H, Bahjat FR, Ungaro R, Debernardis J, Tannahill C, et al. Differential maturation of murine bone-marrow derived dendritic cells with lipopolysaccharide and tumor necrosis factor-α. J Endotoxin Res. 2005; 11:145–60.
Article5). Manzoor Z, Koo JE, Koh YS. Mitogen-activated protein kinase signaling in inflammation-related carcinogenesis. J Bacteriol Virol. 2014; 44:297–304.
Article6). Yuk JM, Jo EK. Toll-like receptors and innate immunity. J Bacteriol Virol. 2011; 41:225–35.
Article7). Akira S, Takeda K. Toll-like receptors signalling. Nat Rev Immunol. 2004; 4:499–511.8). Bae W, Lim HK, Kim KM, Cho H, Lee SY, Jeong CS, et al. Apoptosis-inducing activity of marine sponge haliclona sp. extracts collected from kosrae in nonsmall cell lung cancer A549 cells. Evid Based Complement Alternat Med. 2015. DOI: doi: 10.1155/2015/717959.9). Lordan S, Ross RP, Stanton C. Marine bioactives as functional food ingredients: potential to reduce the incidence of chronic diseases. Mar Drugs. 2011; 9:1056–100.
Article10). Athanasiadis A. Taxonomy of Rhodophyta with particular reference to Mediterranean species. Bocconea. 2003; 16:193–8.11). Liu J, Zhan X, Wan J, Wang Y, Wang C. Review for carrageenan-based pharmaceutical biomaterials: favourable physical features versus adverse biological effects. Carbohydr Polym. 2015; 121:27–36.
Article12). Vijayavel K, Martinez JA. In vitro antioxidant and antimicrobial activities of two Hawaiian marine limu: Ulva fasciata (Chlorophyta) and Gracilaria salicornia (Rhodophyta). J Med Food. 2010; 13:1494–9.13). Manzoor Z, Kim S, Chae D, Yoo ES, Kang HK, Hyun JW, et al. Sea lettuce (Ulva fasciata) extract has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived macrophages and dendritic cells. Food Sci Biotechnol. 2013; 22:781–6.
Article14). Manzoor Z, Mathema VB, Chae D, Yoo ES, Kang HK, Hyun JW, et al. Extracts of the seaweed Sargassum macrocarpum inhibit the CpG-induced inflammatory response by attenuating the NF-κB pathway. Food Sci Biotechnol. 2014; 23:293–7.
Article15). Koo JE, Hong HJ, Dearth A, Kobayashi KS, Koh YS. Intracellular invasion of Orientia tsutsugamushi activates inflammasome in ASC-dependent manner. PLoS ONE. 2012; 7:e39042.
Article16). Chae D, Manzoor Z, Kim SC, Kim S, Oh TH, Yoo ES, et al. Apo-9′-fucoxanthinone, isolated from Sargassum muticum, inhibits CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase pathway. Mar Drugs. 2013; 11:3272–87.
Article17). Koo JE, Hong HJ, Mathema VB, Kang HK, Hyun JW, Kim GY, et al. Inhibitory effects of Carpinus tschonoskii leaves extract on CpG-stimulated pro-inflammatory cytokine production in murine bone marrow-derived macrophages and dendritic cells. In Vitro Cell Dev Biol Anim. 2012; 48:197–202.
Article18). Manzoor Z, Koo JE, Ali I, Kim JE, Byeon SH, Yoo ES, et al. 4-Hydroxy-2, 3-dimethyl-2-nonen-4-olide has an inhibitory effect on pro-inflammatory cytokine production in CpG-stimulated bone marrow-derived dendritic cells. Mar Drugs. 2016. DOI: doi: 10.3390/md14050088.19). Manzoor Z, Mathema VB, Chae D, Kang HK, Yoo ES, Jeon YJ, et al. Octaphlorethol A inhibits the CpG-induced inflammatory response by attenuating the mitogen-activated protein kinase and NF-κB pathways. Biosci Biotechnol Biochem. 2013; 77:1970–2.
Article20). Hsu LC, Park JM, Zhang K, Luo JL, Maeda S, Kaufman RJ, et al. The protein kinase PKR is required for macrophage apoptosis after activation of Toll-like receptor 4. Nature. 2004; 428:341–5.
Article21). Ishii KJ, Koyama S, Nakagawa A, Coban C, Akira S. Host innate immune receptors and beyond: making sense of microbial infections. Cell Host Microbe. 2008; 3:352–63.
Article22). Bao L, Lindgren JU, van der Meide P, Zhu S, Ljunggren HG, Zhu J. The critical role of IL-12p40 in initiating, enhancing, and perpetuating pathogenic events in murine experimental autoimmune neuritis. Brain Pathol. 2002; 12:420–9.
Article23). Gado K, Domjan G, Hegyesi H, Falus A. Role of interleukin-6 in the pathogenesis of multiple myeloma. Cell Biol Int. 2000; 24:195–209.24). Gonzalez S, Rodrigo L, Martinez-Borra J, Lopez-Vazquez A, Fuentes D, Nino P, et al. TNF-alpha -308A promoter polymorphism is associated with enhanced TNF-alpha production and inflammatory activity in Crohn's patients with fistulizing disease. Am J Gastroenterol. 2003; 98:1101–6.25). Guha M, Mackman N. LPS induction of gene expression in human monocytes. Cell Signal. 2001; 13:85–94.
Article26). Nomura F, Akashi S, Sakao Y, Sato S, Kawai T, Matsumoto M, et al. Cutting edge: endotoxin tolerance in mouse peritoneal macrophages correlates with down-regulation of surface toll-like receptor 4 expression. J Immunol. 2000; 164:3476–9.
Article27). Clancy RM, Aminm AR, Abramsonm SB. The role of nitric oxide in inflammation and immunity. Arthritis Rheum. 1998; 41:1141–51.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Estragole Exhibits Anti-inflammatory Activity with the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-induced RAW 264.7 cells
- Anti-inflammatory effects of proanthocyanidin-rich red rice extract via suppression of MAPK, AP-1 and NF-κB pathways in Raw 264.7 macrophages
- 3-Hydroxy-4,7-megastigmadien-9-one, Isolated from Ulva pertusa Kjellman, Inhibits LPS-Induced Inflammatory Response by Down-Regulating Mitogen-Activated Protein Kinase and NF-κB Pathways
- Pulegone Exhibits Anti-inflammatory Activities through the Regulation of NF-κB and Nrf-2 Signaling Pathways in LPS-stimulated RAW 264.7 cells
- p38 MAPK and NF-kappaB are required for LPS-induced RANTES production in immortalized murine microglia (BV-2)