Korean J Physiol Pharmacol.
2016 Sep;20(5):467-476. 10.4196/kjpp.2016.20.5.467.
Effect of pertussis toxin pretreated centrally on blood glucose level induced by stress
- Affiliations
-
- 1Department of Pharmacology, Institute of Natural Medicine, College of Medicine, Hallym University, Chuncheon 24252, Korea.
- 2Adult Stem Cell Research Center in Kangstem Biotech, Seoul National University, Seoul 08826, Korea.
- 3College of Physical Education, Kookmin University, Seoul 02707, Korea.
- 4Department of Physical Education, College of Natural Science, Hallym University, Chuncheon 24252, Korea. jayshong@hallym.ac.kr
- KMID: 2350503
- DOI: http://doi.org/10.4196/kjpp.2016.20.5.467
Abstract
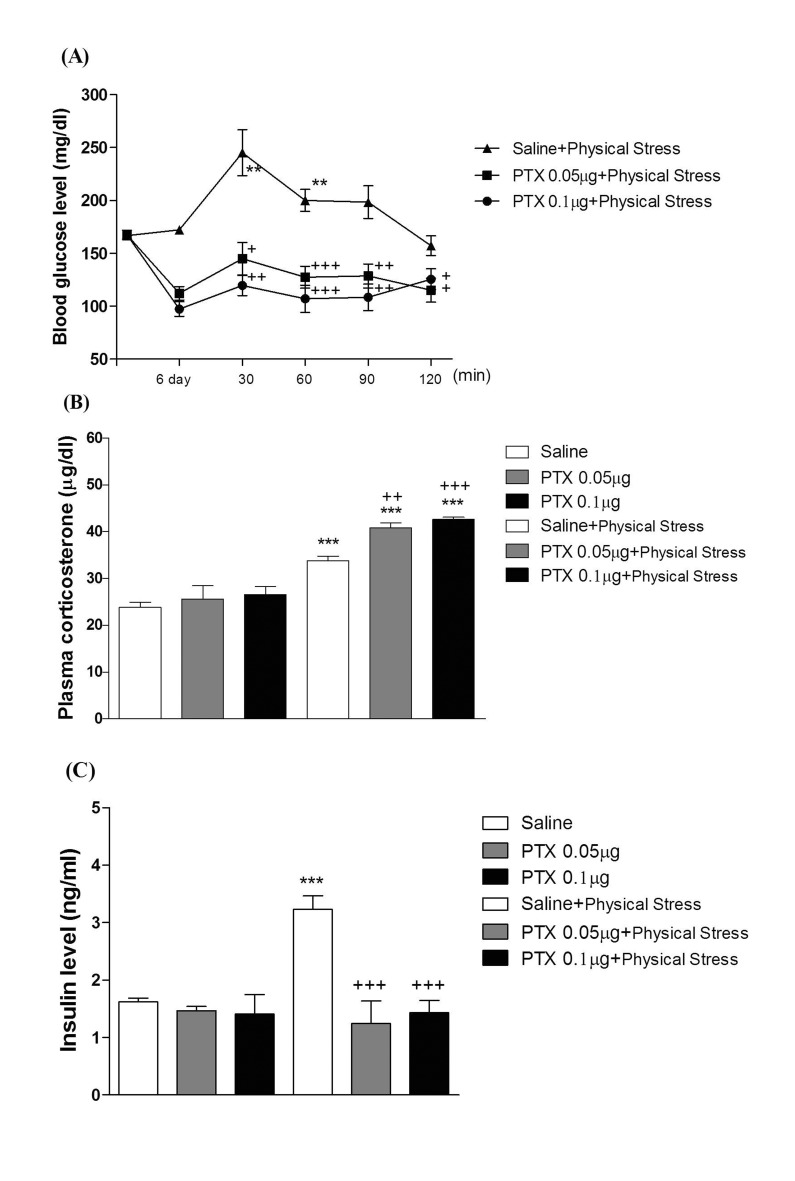
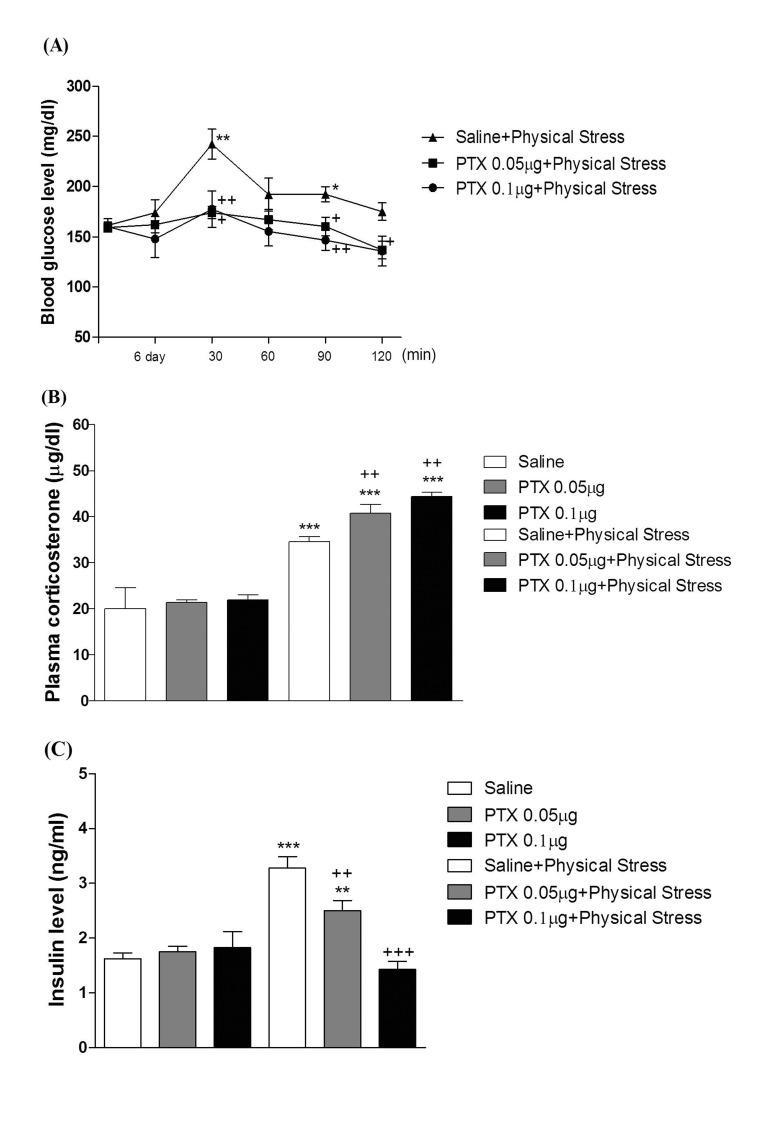
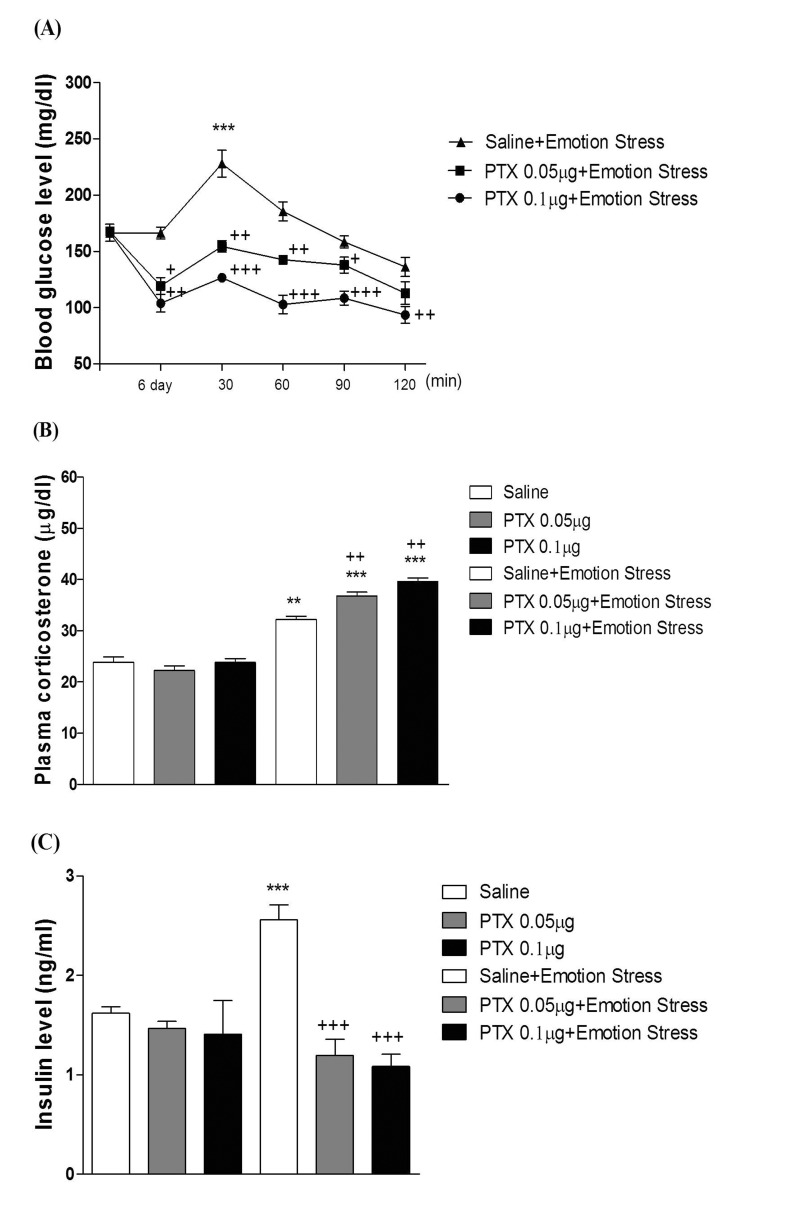
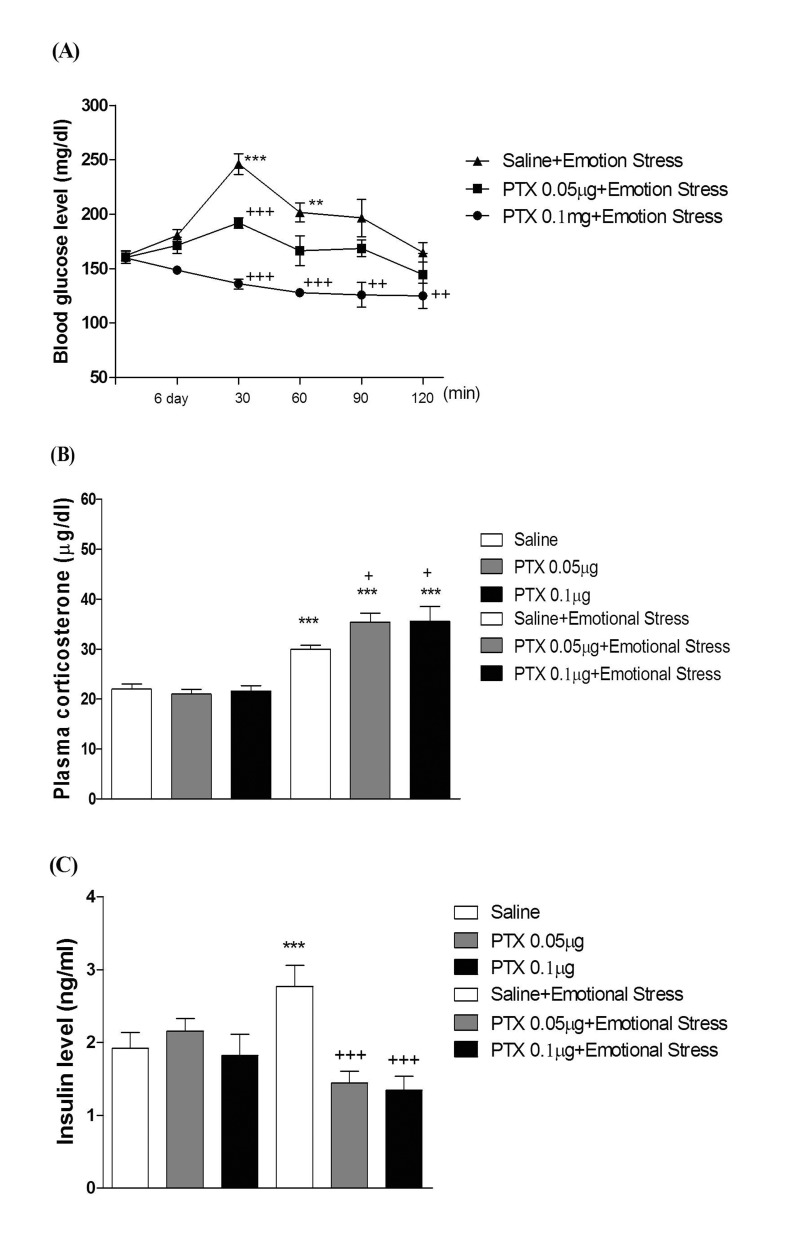
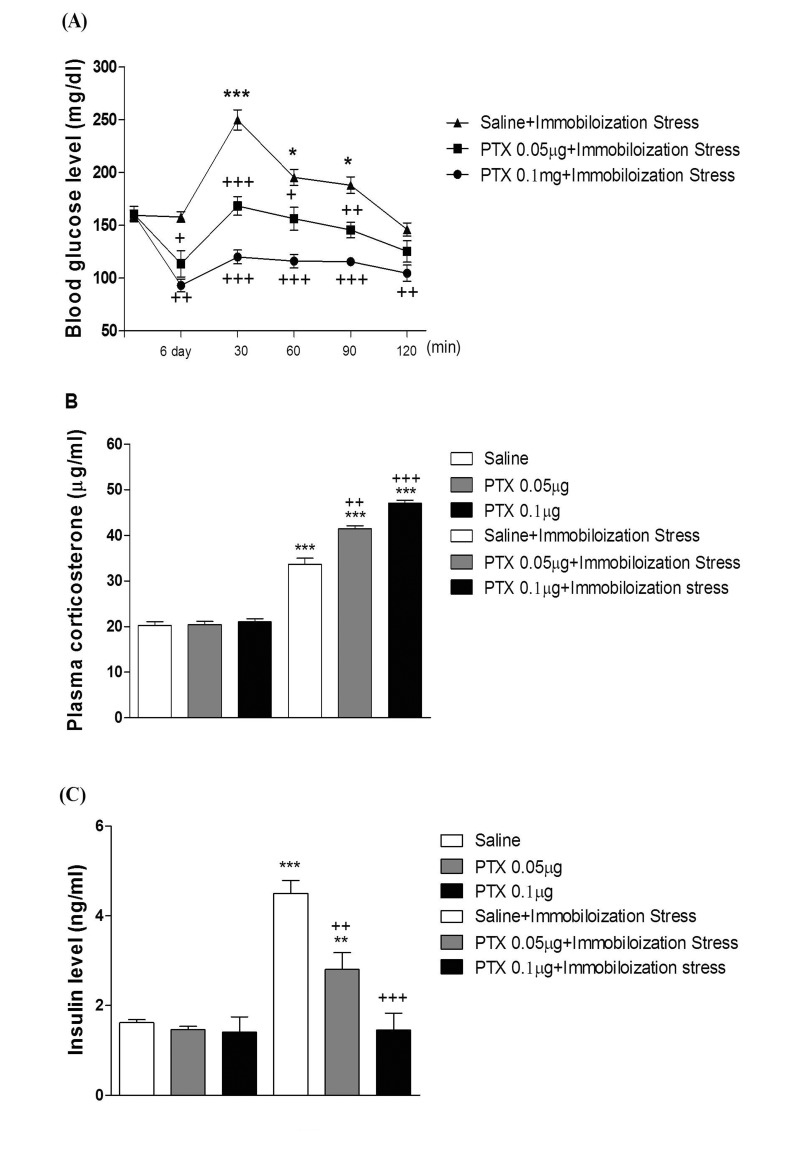
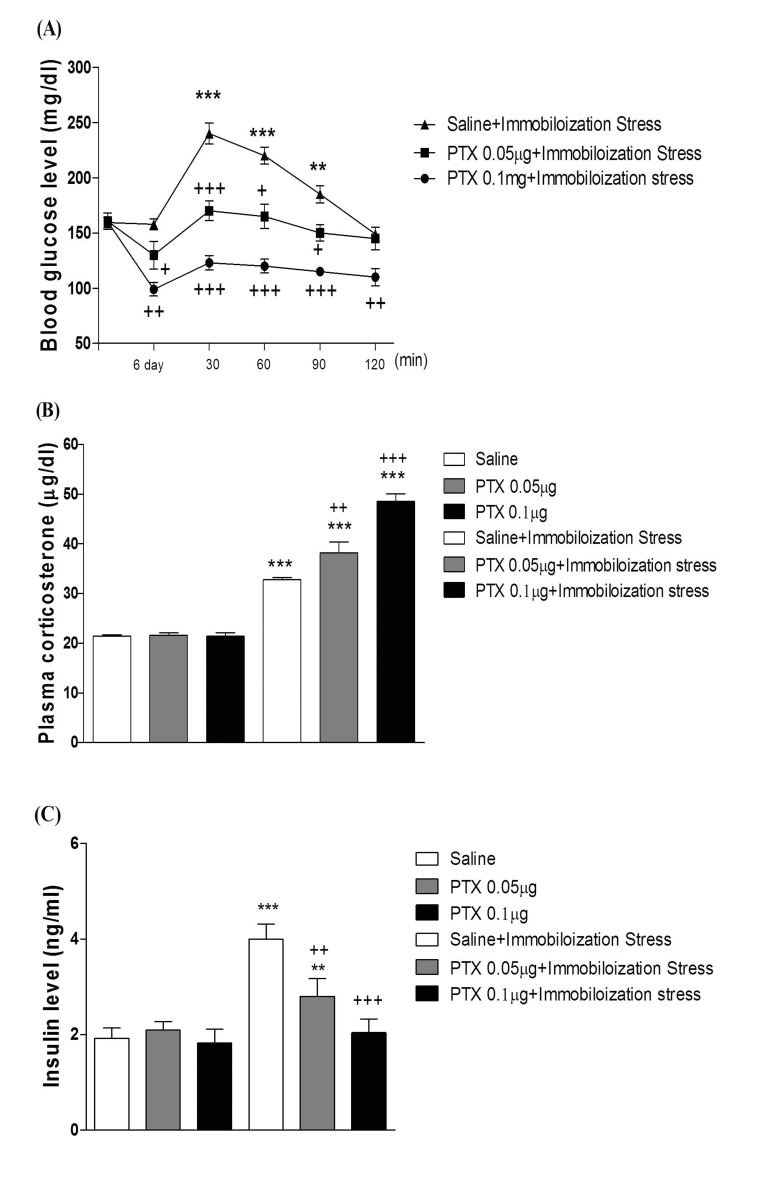
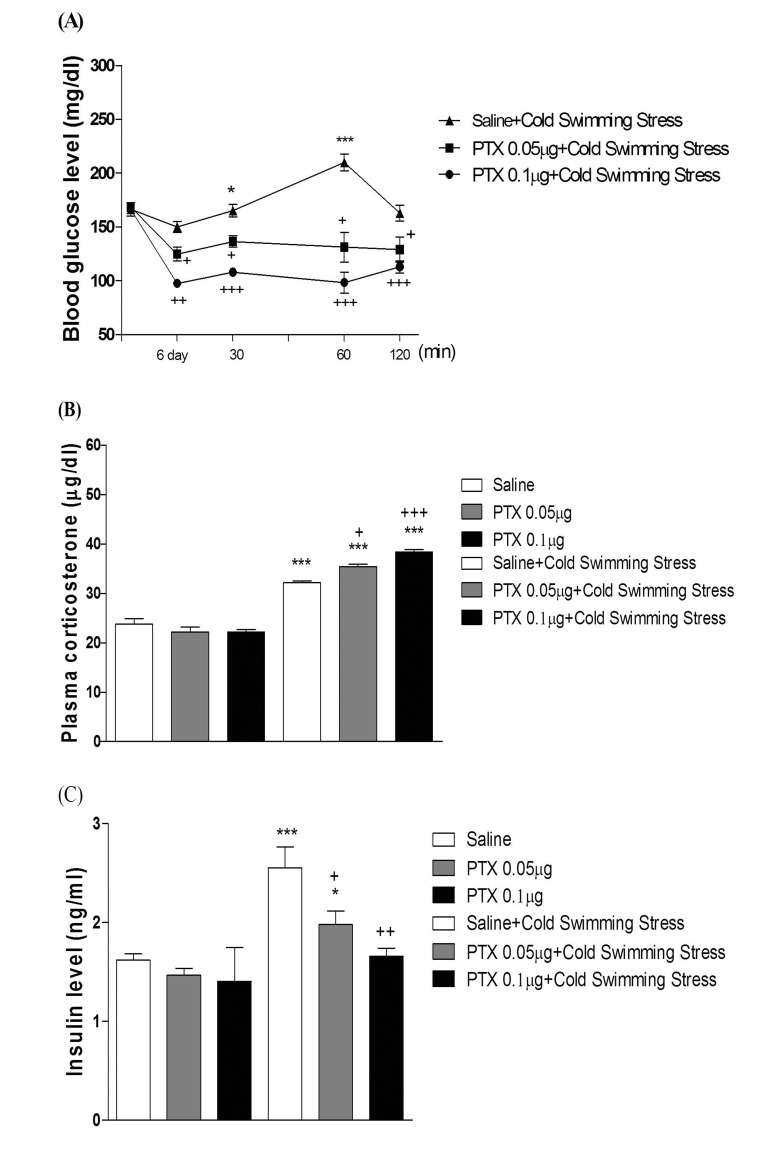
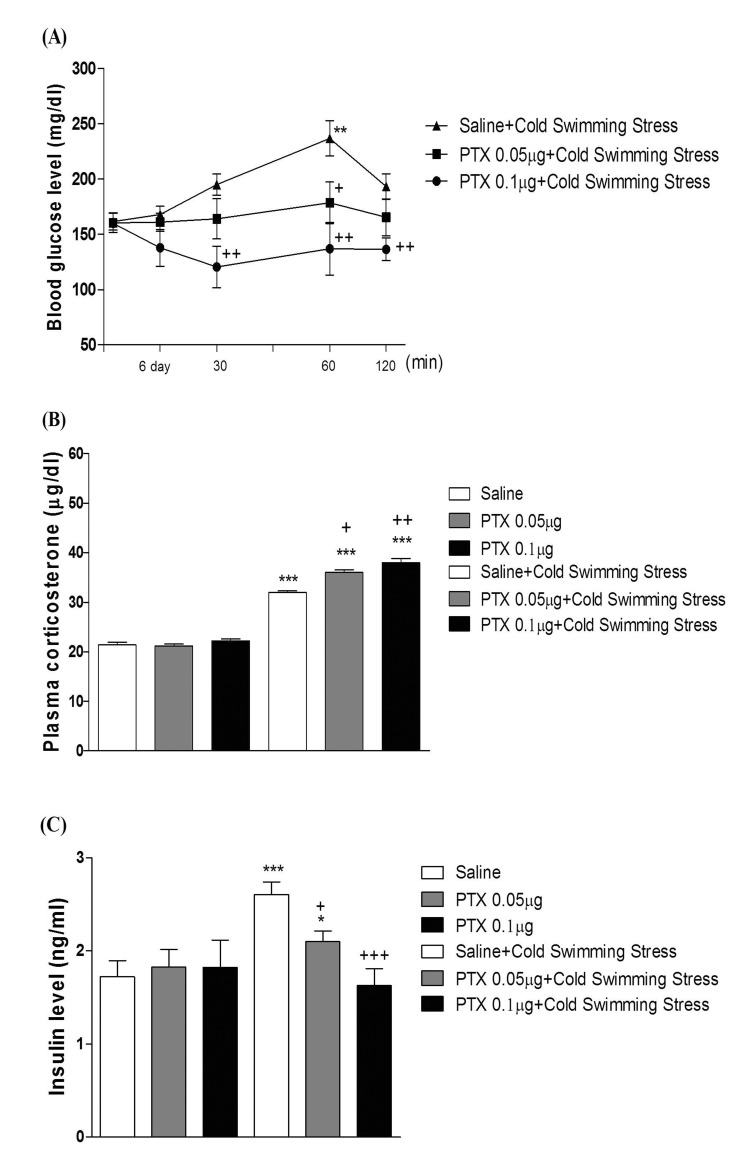
- In the present study, we examined the effect of pertussis toxin (PTX) administered centrally in a variety of stress-induced blood glucose level. Mice were exposed to stress after the pretreatment of PTX (0.05 or 0.1 µg) i.c.v. or i.t. once for 6 days. Blood glucose level was measured at 0, 30, 60 and 120 min after stress stimulation. The blood glucose level was increased in all stress groups. The blood glucose level reached at maximum level after 30 min of stress stimulation and returned to a normal level after 2 h of stress stimulation in restraint stress, physical, and emotional stress groups. The blood glucose level induced by cold-water swimming stress was gradually increased up to 1 h and returned to the normal level. The intracerebroventricular (i.c.v.) or intrathecal (i.t.) pretreatment with PTX, a Gi inhibitor, alone produced a hypoglycemia and almost abolished the elevation of the blood level induced by stress stimulation. The central pretreatment with PTX caused a reduction of plasma insulin level, whereas plasma corticosterone level was further up-regulated in all stress models. Our results suggest that the hyperglycemia produced by physical stress, emotional stress, restraint stress, and the cold-water swimming stress appear to be mediated by activation of centrally located PTX-sensitive G proteins. The reduction of blood glucose level by PTX appears to due to the reduction of plasma insulin level. The reduction of blood glucose level by PTX was accompanied by the reduction of plasma insulin level. Plasma corticosterone level up-regulation by PTX in stress models may be due to a blood glucose homeostatic mechanism.
Keyword
MeSH Terms
Figure
Reference
-
1. Gearhart MM, Parbhoo SK. Hyperglycemia in the critically ill patient. AACN Clin Issues. 2006; 17:50–55. PMID: 16462409.
Article2. Johan Groeneveld AB, Beishuizen A, Visser FC. Insulin: a wonder drug in the critically ill? Crit Care. 2002; 6:102–105. PMID: 11983030.3. van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, Vlasselaers D, Ferdinande P, Lauwers P, Bouillon R. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001; 345:1359–1367. PMID: 11794168.
Article4. McCowen KC, Malhotra A, Bistrian BR. Stress-induced hyperglycemia. Crit Care Clin. 2001; 17:107–124. PMID: 11219223.
Article5. Capes SE, Hunt D, Malmberg K, Gerstein HC. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview. Lancet. 2000; 355:773–778. PMID: 10711923.
Article6. Uresin Y, Erbas B, Ozek M, Ozkök E, Gürol AO. Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J Renin Angiotensin Aldosterone Syst. 2004; 5:93–96. PMID: 15295722.
Article7. Nonogaki K, Iguchi A. Stress, acute hyperglycemia, and hyperlipidemia role of the autonomic nervous system and cytokines. Trends Endocrinol Metab. 1997; 8:192–197. PMID: 18406806.8. Jansen AS, Nguyen XV, Karpitskiy V, Mettenleiter TC, Loewy AD. Central command neurons of the sympathetic nervous system: basis of the fight-or-flight response. Science. 1995; 270:644–646. PMID: 7570024.
Article9. De Boer SF, Koopmans SJ, Slangen JL, Van der Gugten J. Plasma catecholamine, corticosterone and glucose responses to repeated stress in rats: effect of interstressor interval length. Physiol Behav. 1990; 47:1117–1124. PMID: 2395915.
Article10. Natelson BH, Creighton D, McCarty R, Tapp WN, Pitman D, Ottenweller JE. Adrenal hormonal indices of stress in laboratory rats. Physiol Behav. 1987; 39:117–125. PMID: 3562645.
Article11. Natelson BH, Tapp WN, Adamus JE, Mittler JC, Levin BE. Humoral indices of stress in rats. Physiol Behav. 1981; 26:1049–1054. PMID: 7197031.
Article12. Kvetnansky R, Sun CL, Lake CR, Thoa N, Torda T, Kopin IJ. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology. 1978; 103:1868–1874. PMID: 748021.13. Stubbs PJ, Laycock J, Alaghband-Zadeh J, Carter G, Noble MI. Circulating stress hormone and insulin concentrations in acute coronary syndromes: identification of insulin resistance on admission. Clin Sci (Lond). 1999; 96:589–595. PMID: 10334964.
Article14. Ullrich S, Wollheim CB. Islet cyclic AMP levels are not lowered during alpha 2-adrenergic inhibition of insulin release. J Biol Chem. 1984; 259:4111–4115. PMID: 6323458.
Article15. Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, Straub RH, Paus R. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. FASEB J. 2005; 19:1332–1334. PMID: 15946990.16. Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010; 85:33–39. PMID: 20412987.
Article17. Fagerholm V, Haaparanta M, Scheinin M. α2-adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol. 2011; 108:365–370. PMID: 21418144.
Article18. Sapolsky RM. Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry. 2000; 57:925–935. PMID: 11015810.
Article19. Hoxworth JM, Xu K, Zhou Y, Lust WD, LaManna JC. Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res. 1999; 821:467–479. PMID: 10064834.
Article20. Anderson RE, Tan WK, Martin HS, Meyer FB. Effects of glucose and PaO2 modulation on cortical intracellular acidosis, NADH redox state, and infarction in the ischemic penumbra. Stroke. 1999; 30:160–170. PMID: 9880405.21. Siesjö BK, Katsura KI, Kristián T, Li PA, Siesjö P. Molecular mechanisms of acidosis-mediated damage. Acta Neurochir Suppl. 1996; 66:8–14. PMID: 8780790.
Article22. Ploug T, Han X, Petersen LN, Galbo H. Effect of in vivo injection of cholera and pertussis toxin on glucose transport in rat skeletal muscle. Am J Physiol. 1997; 272:E7–E17. PMID: 9038845.
Article23. Ciaraldi TP, Maisel A. Role of guanine nucleotide regulatory proteins in insulin stimulation of glucose transport in rat adipocytes Influence of bacterial toxins. Biochem J. 1989; 264:389–396. PMID: 2557836.
Article24. Kim CH, Park SH, Sim YB, Sharma N, Kim SS, Lim SM, Jung JS, Suh HW. Effect of pertussis and cholera toxins administered supraspinally on CA3 hippocampal neuronal cell death and the blood glucose level induced by kainic acid in mice. Neurosci Res. 2014; 89:31–36. PMID: 25218563.
Article25. Sim YB, Park SH, Kim SS, Lim SM, Jung JS, Lee JK, Suh HW. Pertussis toxin administered spinally induces a hypoglycemic effect on normal and diabetic mice. Pharmacology. 2014; 94:29–40. PMID: 25171426.
Article26. Laursen SE, Belknap JK. Intracerebroventricular injections in mice Some methodological refinements. J Pharmacol Methods. 1986; 16:355–357. PMID: 3784576.27. Hylden JL, Wilcox GL. Intrathecal substance P elicits a caudally-directed biting and scratching behavior in mice. Brain Res. 1981; 217:212–215. PMID: 6167328.
Article28. Van den Berg CL, Lamberts RR, Wolterink G, Wiegant VM, Van Ree JM. Emotional and footshock stimuli induce differential long-lasting behavioural effects in rats; involvement of opioids. Brain Res. 1998; 799:6–15. PMID: 9666058.
Article29. Suh HW, Song DK, Huh SO, Kim YH. Involvement of dynorphin in immobilization stress-induced antinociception in the mouse. Eur Neuropsychopharmacol. 2000; 10:407–413. PMID: 10974614.
Article30. Glick G, Plauth WH Jr, Braunwald E. Role of the autonomic nervous system in the circulatory response to acutely induced anemia in unanesthetized dogs. J Clin Invest. 1964; 43:2112–2124. PMID: 14223923.31. Sharma N, Sim YB, Park SH, Lim SM, Kim SS, Jung JS, Hong JS, Suh HW. Effect of sulfonylureas administered centrally on the blood glucose level in immobilization stress model. Korean J Physiol Pharmacol. 2015; 19:197–202. PMID: 25954123.
Article32. Kang YJ, Sim YB, Park SH, Sharma N, Suh HW. Involvement of α(2)-adrenergic receptor in the regulation of the blood glucose level induced by immobilization stress. Arch Pharm Res. 2015; 38:921–929. PMID: 24993869.
Article33. Marcovecchio ML, Chiarelli F. The effects of acute and chronic stress on diabetes control. Sci Signal. 2012; 5:pt10. PMID: 23092890.34. Sim YB, Park SH, Kang YJ, Kim SM, Lee JK, Jung JS, Suh HW. The regulation of blood glucose level in physical and emotional stress models: possible involvement of adrenergic and glucocorticoid systems. Arch Pharm Res. 2010; 33:1679–1683. PMID: 21052944.
Article35. Amano M, Suemaru K, Cui R, Umeda Y, Li B, Gomita Y, Kawasaki H, Araki H. Effects of physical and psychological stress on 5-HT2A receptor-mediated wet-dog shake responses in streptozotocin-induced diabetic rats. Acta Med Okayama. 2007; 61:205–212. PMID: 17726509.36. Márquez C, Belda X, Armario A. Post-stress recovery of pituitary-adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Res. 2002; 926:181–185. PMID: 11814422.
Article37. Verago JL, Grassi-Kassisse DM, Spadari-Bratfisch RC. Metabolic markers following beta-adrenoceptor agonist infusion in footshock-stressed rats. Braz J Med Biol Res. 2001; 34:1197–1207. PMID: 11514845.
Article38. Dobrakovová M, Kvetnanský R, Oprsalová Z, Jezová D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993; 18:163–174. PMID: 8390698.
Article39. Konarska M, Stewart RE, McCarty R. Habituation of sympathetic-adrenal medullary responses following exposure to chronic intermittent stress. Physiol Behav. 1989; 45:255–261. PMID: 2756012.
Article40. Cox RH, Hubbard JW, Lawler JE, Sanders BJ, Mitchell VP. Cardiovascular and sympathoadrenal responses to stress in swim-trained rats. J Appl Physiol (1985). 1985; 58:1207–1214. PMID: 3988676.
Article41. Sim YB, Park SH, Kim SS, Lim SM, Jung JS, Suh HW. The modulatory role of alpha-melanocyte stimulating hormone administered spinally in the regulation of blood glucose level in d-glucose-fed and restraint stress mouse models. Neuropeptides. 2014; 48:207–212. PMID: 24912936.
Article42. Sim YB, Park SH, Kang YJ, Kim SS, Kim CH, Kim SJ, Jung JS, Ryu OH, Choi MG, Suh HW. Effect of GABA receptor agonists or antagonists injected spinally on the blood glucose level in mice. Neurochem Res. 2013; 38:1055–1062. PMID: 23508311.
Article43. Sim YB, Park SH, Kim SS, Lim SM, Jung JS, Suh HW. Activation of spinal α2 adrenergic receptors induces hyperglycemia in mouse though activating sympathetic outflow. Eur J Pharmacol. 2014; 741:316–322. PMID: 25179570.
Article44. Kainuma E, Watanabe M, Tomiyama-Miyaji C, Inoue M, Kuwano Y, Ren H, Abo T. Association of glucocorticoid with stress-induced modulation of body temperature, blood glucose and innate immuni ty. Psychoneuroendocrinology. 2009; 34:1459–1468. PMID: 19493627.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Sulfonylureas Administered Centrally on the Blood Glucose Level in Immobilization Stress Model
- Effect of D-glucose feeding on mortality induced by sepsis
- The activation of α₂-adrenergic receptor in the spinal cord lowers sepsis-induced mortality
- Effects of Pertussis Toxin on the Differentiation of B Lymphocytes in Lymph Node
- Intracerebroventricular Kainic Acid-Induced Damage Affects Blood Glucose Level in d-glucose-fed Mouse Model