Korean J Physiol Pharmacol.
2015 May;19(3):197-202. 10.4196/kjpp.2015.19.3.197.
Effect of Sulfonylureas Administered Centrally on the Blood Glucose Level in Immobilization Stress Model
- Affiliations
-
- 1Department of Pharmacology, Institute of Natural Medicine, Hallym University, Chuncheon 200-702, Korea. hwsuh@hallym.ac.kr
- 2Department of Physical Education, College of Natural Medicine, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- KMID: 1791419
- DOI: http://doi.org/10.4196/kjpp.2015.19.3.197
Abstract
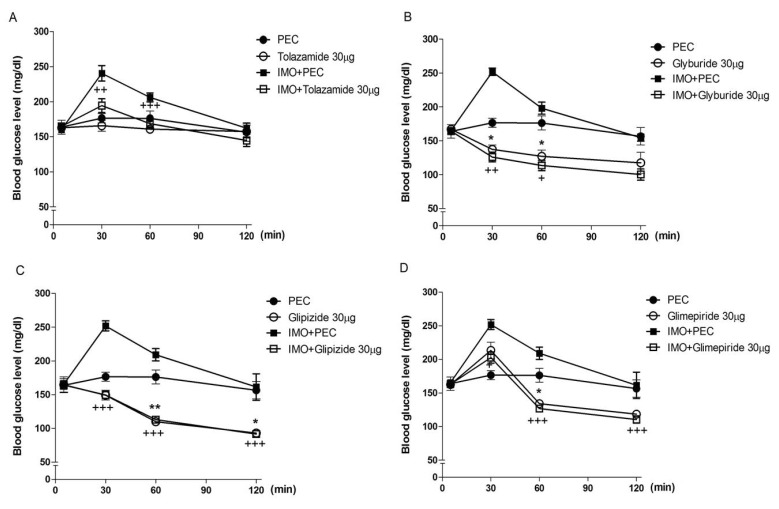
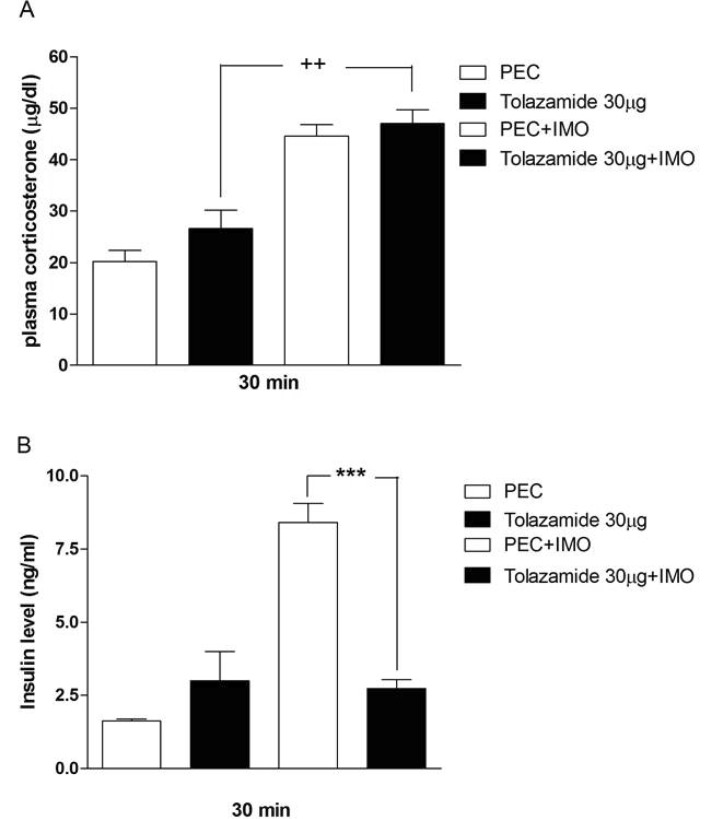
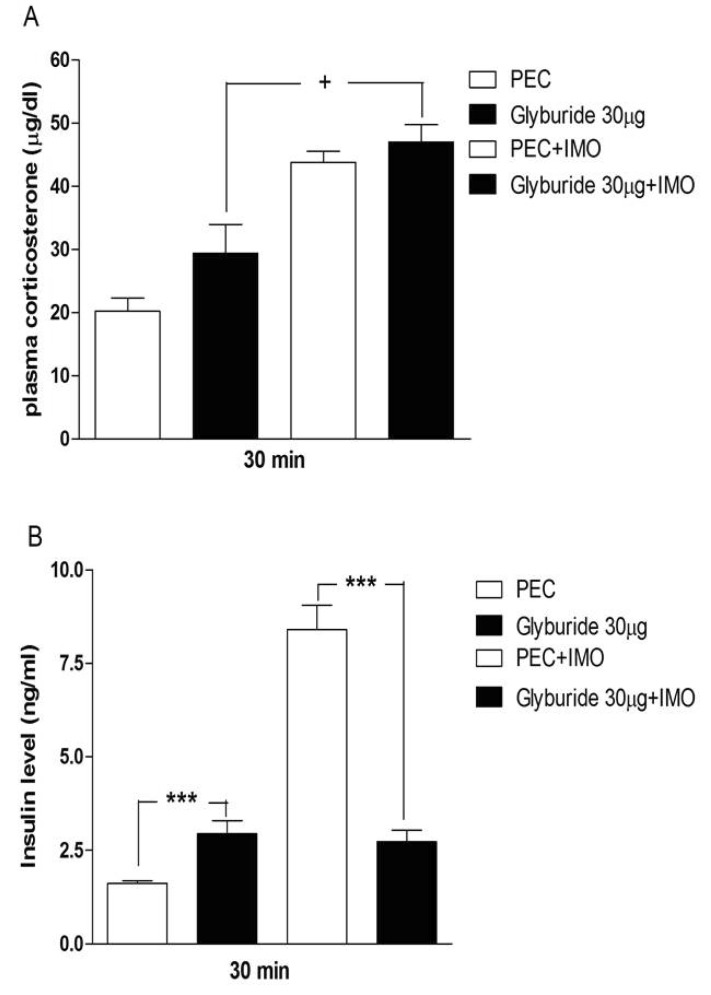
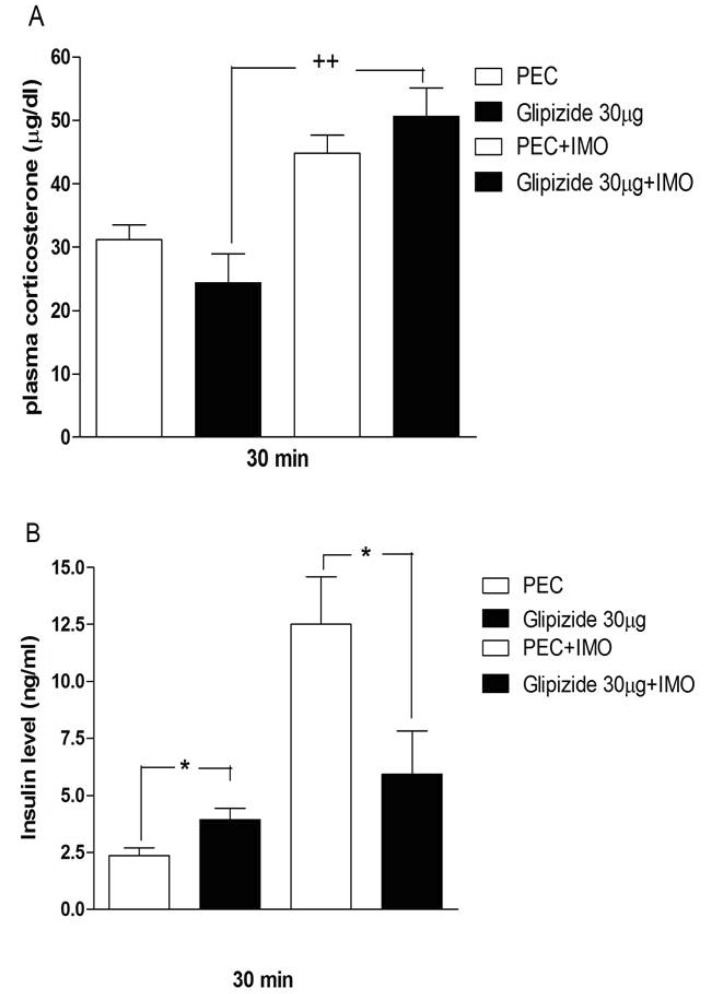
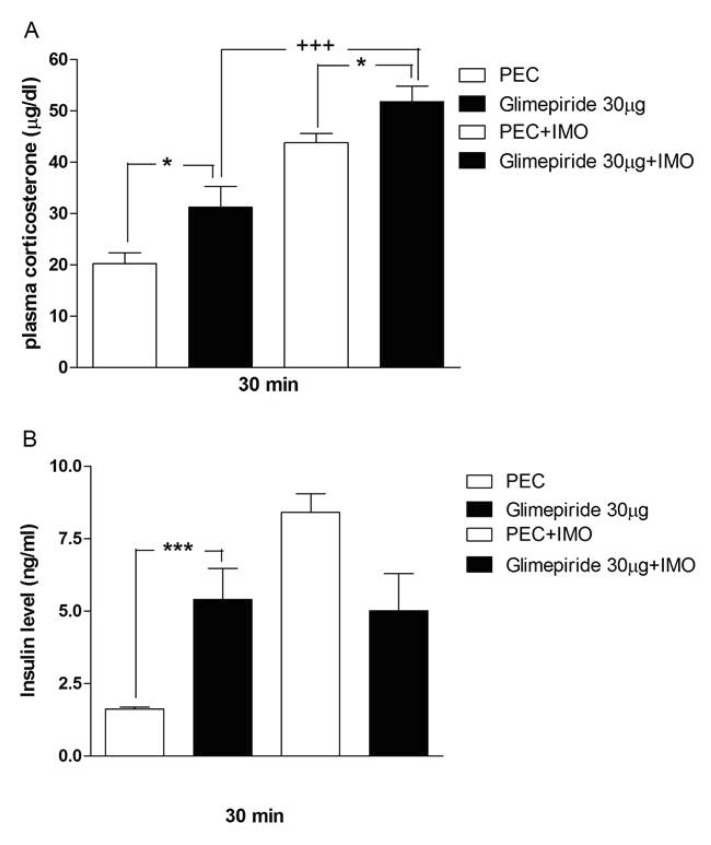
- Sulfonylureas are widely used as an antidiabetic drug. In the present study, the effects of sulfonylurea administered supraspinally on immobilization stress-induced blood glucose level were studied in ICR mice. Mice were once enforced into immobilization stress for 30 min and returned to the cage. The blood glucose level was measured 30, 60, and 120 min after immobilization stress initiation. We found that intracerebroventricular (i.c.v.) injection with 30 microg of glyburide, glipizide, glimepiride or tolazamide attenuated the increased blood glucose level induced by immobilization stress. Immobilization stress causes an elevation of the blood corticosterone and insulin levels. Sulfonylureas pretreated i.c.v. caused a further elevation of the blood corticosterone level when mice were forced into the stress. In addition, sulfonylureas pretreated i.c.v. alone caused an elevation of the plasma insulin level. Furthermore, immobilization stress-induced insulin level was reduced by i.c.v. pretreated sulfonylureas. Our results suggest that lowering effect of sulfonylureas administered supraspinally against immobilization stress-induced increase of the blood glucose level appears to be primarily mediated via elevation of the plasma insulin level.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Effect of pertussis toxin pretreated centrally on blood glucose level induced by stress
Hong-Won Suh, Yun-Beom Sim, Soo-Hyun Park, Naveen Sharma, Hyun-Ju Im, Jae-Seung Hong
Korean J Physiol Pharmacol. 2016;20(5):467-476. doi: 10.4196/kjpp.2016.20.5.467.
Reference
-
1. Chen Y, Kramár EA, Chen LY, Babayan AH, Andres AL, Gall CM, Lynch G, Baram TZ. Impairment of synaptic plasticity by the stress mediator CRH involves selective destruction of thin dendritic spines via RhoA signaling. Mol Psychiatry. 2013; 18:485–496. PMID: 22411227.
Article2. Glavin GB, Paré WP, Sandbak T, Bakke HK, Murison R. Restraint stress in biomedical research: an update. Neurosci Biobehav Rev. 1994; 18:223–249. PMID: 8058215.
Article3. Honkaniemi J. Colocalization of peptide- and tyrosine hydroxylase-like immunoreactivities with Fos-immunoreactive neurons in rat central amygdaloid nucleus after immobilization stress. Brain Res. 1992; 598:107–113. PMID: 1362516.
Article4. Honkaniemi J, Kainu T, Ceccatelli S, Rechardt L, Hökfelt T, Pelto-Huikko M. Fos and jun in rat central amygdaloid nucleus and paraventricular nucleus after stress. Neuroreport. 1992; 3:849–852. PMID: 1421086.
Article5. Seo YJ, Kwon MS, Shim EJ, Park SH, Choi OS, Suh HW. Changes in pain behavior induced by formalin, substance P, glutamate and pro-inflammatory cytokines in immobilizationinduced stress mouse model. Brain Res Bull. 2006; 71:279–286. PMID: 17113957.
Article6. Surwit RS, Schneider MS, Feinglos MN. Stress and diabetes mellitus. Diabetes Care. 1992; 15:1413–1422. PMID: 1425110.
Article7. Gądek-Michalska A, Tadeusz J, Rachwalska P, Spyrka J, Bugajski J. Effect of prior stress on interleukin-1β and HPA axis responses to acute stress. Pharmacol Rep. 2011; 63:1393–1403. PMID: 22358087.8. Uresin Y, Erbas B, Ozek M, Ozkök E, Gürol AO. Losartan may prevent the elevation of plasma glucose, corticosterone and catecholamine levels induced by chronic stress. J Renin Angiotensin Aldosterone Syst. 2004; 5:93–96. PMID: 15295722.
Article9. Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos GP. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010; 85:33–39. PMID: 20412987.
Article10. Fagerholm V, Haaparanta M, Scheinin M. α2-adrenoceptor regulation of blood glucose homeostasis. Basic Clin Pharmacol Toxicol. 2011; 108:365–370. PMID: 21418144.
Article12. Lebovitz HE, Feinglos MN. Sulfonylurea drugs: mechanism of antidiabetic action and therapeutic usefulness. Diabetes Care. 1978; 1:189–198. PMID: 365478.
Article13. Groop L, Wåhlin-Boll E, Groop PH, Tötterman KJ, Melander A, Tolppanen EM, Fyhrqvist F. Pharmacokinetics and metabolic effects of glibenclamide and glipizide in type 2 diabetics. Eur J Clin Pharmacol. 1985; 28:697–704. PMID: 3933984.
Article14. Skillman TG, Feldman JM. The pharmacology of sulfonylureas. Am J Med. 1981; 70:361–372. PMID: 6781341.
Article15. Chari M, Lam CK, Wang PY, Lam TK. Activation of central lactate metabolism lowers glucose production in uncontrolled diabetes and diet-induced insulin resistance. Diabetes. 2008; 57:836–840. PMID: 18184925.
Article16. Park SH, Sim YB, Kang YJ, Kim SM, Lee JK, Jung JS, Suh HW. Characterization of blood glucose level regulation in mouse opioid withdrawal models. Neurosci Lett. 2010; 476:119–122. PMID: 20226840.
Article17. Sim YB, Park SH, Kang YJ, Kim SS, Kim CH, Kim SJ, Lim SM, Jung JS, Ryu OH, Choi MG, Suh HW. Repaglinide, but not nateglinide administered supraspinally and spinally exerts an anti-diabetic action in d-glucose fed and streptozotocintreated mouse models. Korean J Physiol Pharmacol. 2013; 17:493–497. PMID: 24381497.
Article18. Haley TJ. Pharmacological effects produced by intracerebral administration of drugs of unrelated structure to conscious mice. Arch Int Pharmacodyn Ther. 1957; 110:239–244. PMID: 13435953.19. Suh HW, Song DK, Huh SO, Kim YH. Involvement of dynorphin in immobilization stress-induced antinociception in the mouse. Eur Neuropsychopharmacol. 2000; 10:407–413. PMID: 10974614.
Article20. Glick D, Vonredlich D, Levine S. Fluorometric determination of corticosterone and cortisol in 0.02-0.05 milliliters of plasma or submilligram samples of adrenal tissue. Endocrinology. 1964; 74:653–655. PMID: 14183213.21. Kim JG, Jung HS, Kim KJ, Min SS, Yoon BJ. Basal blood corticosterone level is correlated with susceptibility to chronic restraint stress in mice. Neurosci Lett. 2013; 555:137–142. PMID: 24064064.
Article22. Nooshinfar E, Akbarzadeh-Baghban A, Meisami E. Effects of increasing durations of immobilization stress on plasma corticosterone level, learning and memory and hippocampal BDNF gene expression in rats. Neurosci Lett. 2011; 500:63–66. PMID: 21683767.
Article23. Pitman DL, Ottenweller JE, Natelson BH. Plasma corticosterone levels during repeated presentation of two intensities of restraint stress: chronic stress and habituation. Physiol Behav. 1988; 43:47–55. PMID: 3413250.
Article24. Magariños AM, Verdugo JM, McEwen BS. Chronic stress alters synaptic terminal structure in hippocampus. Proc Natl Acad Sci U S A. 1997; 94:14002–14008. PMID: 9391142.25. Ricart-Jané D, Rodríguez-Sureda V, Benavides A, Peinado-Onsurbe J, López-Tejero MD, Llobera M. Immobilization stress alters intermediate metabolism and circulating lipoproteins in the rat. Metabolism. 2002; 51:925–931. PMID: 12077743.
Article26. Müller G, Wied S. The sulfonylurea drug, glimepiride, stimulates glucose transport, glucose transporter translocation, and dephosphorylation in insulin-resistant rat adipocytes in vitro. Diabetes. 1993; 42:1852–1867. PMID: 8243832.
Article27. Bernardi H, De Weille JR, Epelbaum J, Mourre C, Amoroso S, Slama A, Fosset M, Lazdunski M. ATP-modulated K+ channels sensitive to antidiabetic sulfonylureas are present in adenohypophysis and are involved in growth hormone release. Proc Natl Acad Sci U S A. 1993; 90:1340–1344. PMID: 8433992.28. Levin BE, Brown KL, Dunn-Meynell AA. Differential effects of diet and obesity on high and low affinity sulfonylurea binding sites in the rat brain. Brain Res. 1996; 739:293–300. PMID: 8955950.
Article29. Amoroso S, Schmid-Antomarchi H, Fosset M, Lazdunski M. Glucose, sulfonylureas, and neurotransmitter release: role of ATP-sensitive K+ channels. Science. 1990; 247:852–854. PMID: 2305257.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of pertussis toxin pretreated centrally on blood glucose level induced by stress
- Repaglinide, but Not Nateglinide Administered Supraspinally and Spinally Exerts an Anti-Diabetic Action in D-Glucose Fed and Streptozotocin-Treated Mouse Models
- The Molecular Signatures of Acute-immobilization-induced Antinociception and Chronic-immobilization-induced Antinociceptive Tolerance
- Effect of Cholera Toxin Administered Supraspinally or Spinally on the Blood Glucose Level in Pain and D-Glucose Fed Animal Models
- Effect of lithium on the Polyamine Response in Brain after Stress