Korean Circ J.
2016 May;46(3):335-342. 10.4070/kcj.2016.46.3.335.
Effects of Angiotensin-II Receptor Blocker on Inhibition of Thrombogenicity in a Canine Atrial Fibrillation Model
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Korea University College of Medicine, Korea University Medical Center, Seoul, Korea. jongilchoi@korea.ac.kr
- 2Department of Thoracic and Cardiovascular Surgery, Korea University College of Medicine, Korea University Medical Center, Seoul, Korea.
- 3Kim Min Kyung's Pathology Clinic, Seoul, Korea.
- KMID: 2344446
- DOI: http://doi.org/10.4070/kcj.2016.46.3.335
Abstract
- BACKGROUND AND OBJECTIVES
Angiotensin-II receptor blockers (ARBs) are known to reduce the development of atrial fibrillation (AF) through reverse-remodeling. However, the effect of ARBs on thrombogenicity in AF remains unknown.
MATERIALS AND METHODS
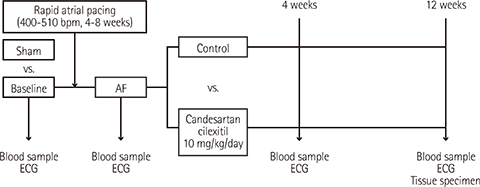
Twelve dogs were assigned to control (n=4), ARB (candesartan cilexitil 10 mg/kg/day p.o., 12 weeks; n=4), or sham (n=4) groups. Sustained AF was induced by rapid atrial pacing. Both arterial and venous serum levels of tissue inhibitor of matrix metalloproteinase-1, von Willebrand factor, P-selectin, and vascular cell adhesion molecule-1 (VCAM-1) were measured at baseline and during AF (0, 4, and 12 weeks) with enzyme-linked immunosorbent assay. Biopsies from both atria including the appendages were performed to semi-quantitatively assess endocardial and myocardial fibrosis after 12 weeks.
RESULTS
The serum levels of bio-markers were not significantly different at baseline or during AF between the control and the candesartan groups. The levels were not significantly different over time, but there was a trend toward a decrease in arterial VCAM-1 from 4 to 12 weeks in the candesartan group compared to the control group. The grades of endocardial fibrosis after 12 weeks but not those of myocardial fibrosis were slightly reduced in the candesartan group compared to the control group.
CONCLUSION
This study did not show that the ARB candesartan significantly reverses thrombogenicity or fibrosis during AF. Future studies using a larger number of subjects are warranted to determine the therapeutic effect of renin-angiotensin-aldosterone system blockade on prothrombogenic processes in AF.
MeSH Terms
-
Angiotensin II
Animals
Atrial Fibrillation*
Biomarkers
Biopsy
Dogs
Enzyme-Linked Immunosorbent Assay
Fibrosis
Matrix Metalloproteinase 1
P-Selectin
Renin-Angiotensin System
Thromboembolism
Vascular Cell Adhesion Molecule-1
von Willebrand Factor
Angiotensin II
Biomarkers
Matrix Metalloproteinase 1
P-Selectin
Vascular Cell Adhesion Molecule-1
von Willebrand Factor
Figure
Reference
-
1. Stewart S, Murphy NF, Walker A, McGuire A, McMurray JJ. Cost of an emerging epidemic: an economic analysis of atrial fibrillation in the UK. Heart. 2004; 90:286–292.2. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res. 2002; 54:230–246.3. Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008; 51:802–809.4. Goette A, Bukowska A, Lendeckel U, et al. Angiotensin II receptor blockade reduces tachycardia-induced atrial adhesion molecule expression. Circulation. 2008; 117:732–742.5. Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow's triad revisited. Lancet. 2009; 373:155–166.6. Nakashima H, Kumagai K. Reverse-remodeling effects of angiotensin II type 1 receptor blocker in a canine atrial fibrillation model. Circ J. 2007; 71:1977–1982.7. Kumagai K, Nakashima H, Urata H, Gondo N, Arakawa K, Saku K. Effects of angiotensin II type 1 receptor antagonist on electrical and structural remodeling in atrial fibrillation. J Am Coll Cardiol. 2003; 41:2197–2204.8. Vasiljević JD, Popović ZB, Otašević P, et al. Myocardial fibrosis assessment by semiquantitative, point-counting and computer-based methods in patients with heart muscle disease: a comparative study. Histopathology. 2001; 38:338–343.9. Nattel S. Defining "culprit mechanisms" in arrhythmogenic cardiac remodeling. Circ Res. 2004; 94:1403–1405.10. Everett TH 4th, Olgin JE. Atrial fibrosis and the mechanisms of atrial fibrillation. Heart Rhythm. 2007; 4:Suppl. S24–S27.11. Freestone B, Beevers DG, Lip GY. The renin-angiotensin-aldosterone system in atrial fibrillation: a new therapeutic target? J Hum Hypertens. 2004; 18:461–465.12. Schotten U, Verheule S, Kirchhof P, Goette A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev. 2011; 91:265–325.13. Lin CS, Pan CH. Regulatory mechanisms of atrial fibrotic remodeling in atrial fibrillation. Cell Mol Life Sci. 2008; 65:1489–1508.14. Li D, Shinagawa K, Pang L, et al. Effects of angiotensin-converting enzyme inhibition on the development of the atrial fibrillation substrate in dogs with ventricular tachypacing-induced congestive heart failure. Circulation. 2001; 104:2608–2614.15. He X, Gao X, Peng L, et al. Atrial fibrillation induces myocardial fibrosis through angiotensin II type 1 receptor-specific Arkadia-mediated downregulation of Smad7. Circ Res. 2011; 108:164–175.16. Kim HS, No CW, Goo SH, et al. An angiotensin receptor blocker prevents arrhythmogenic left atrial remodeling in a rat post myocardial infarction induced heart failure model. J Korean Med Sci. 2013; 28:700–708.17. Park JH, Lee JS, Ko YG, et al. Histological and biochemical comparisons between right atrium and left atrium in patients with mitral valvular atrial fibrillation. Korean Circ J. 2014; 44:233–242.18. Das UN. Is angiotensin-II an endogenous pro-inflammatory molecule? Med Sci Monit. 2005; 11:RA155–RA162.19. Takagishi T, Murahashi N, Azagami S, et al. Effect of angiotensin II and thromboxane A2 on the production of matrix metalloproteinase by human aortic smooth muscle cells. Biochem Mol Biol Int. 1995; 35:265–273.20. Wachtell K, Hornestam B, Lehto M, et al. Cardiovascular morbidity and mortality in hypertensive patients with a history of atrial fibrillation: The Losartan Intervention For End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005; 45:705–711.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lithium Intoxication Induced by Angiotensin II Receptor Blocker/Thiazide Combination Agent
- Current Issues on the Angiotensin II Receptor Blocker in Cardiovascular Disease
- Preventive Effects of the Angiotensin-II Receptor Blocker on Atrial Remodeling in an Ischemic Heart Failure Model of Rats
- Angiotensin Receptor Blocker for Stroke Prevention in Atrial Fibrillation: beyond Blood Pressure Lowering?
- An Angiotensin Receptor Blocker Prevents Arrhythmogenic Left Atrial Remodeling in a Rat Post Myocardial Infarction Induced Heart Failure Model