Korean J Radiol.
2015 Dec;16(6):1283-1293. 10.3348/kjr.2015.16.6.1283.
The Safety and Clinical Outcomes of Chemoembolization in Child-Pugh Class C Patients with Hepatocellular Carcinomas
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, Seoul National University Hospital, Seoul 03080, Korea. angiointervention@gmail.com
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul 03080, Korea.
- KMID: 2344283
- DOI: http://doi.org/10.3348/kjr.2015.16.6.1283
Abstract
OBJECTIVE
To evaluate the safety and clinical outcomes of chemoembolization in Child-Pugh class C patients with hepatocellular carcinomas (HCC).
MATERIALS AND METHODS
The study comprised 55 patients with HCC who were classified as Child-Pugh class C and who underwent initial chemoembolization between January 2003 and December 2012. Selective chemoembolization was performed in all technically feasible cases to minimize procedure-related complications. All adverse events within 30 days were recorded using the Common Terminology Criteria for Adverse Events (CTCAE). The tumor response to chemoembolization was evaluated using the modified Response Evaluation Criteria In Solid Tumors.
RESULTS
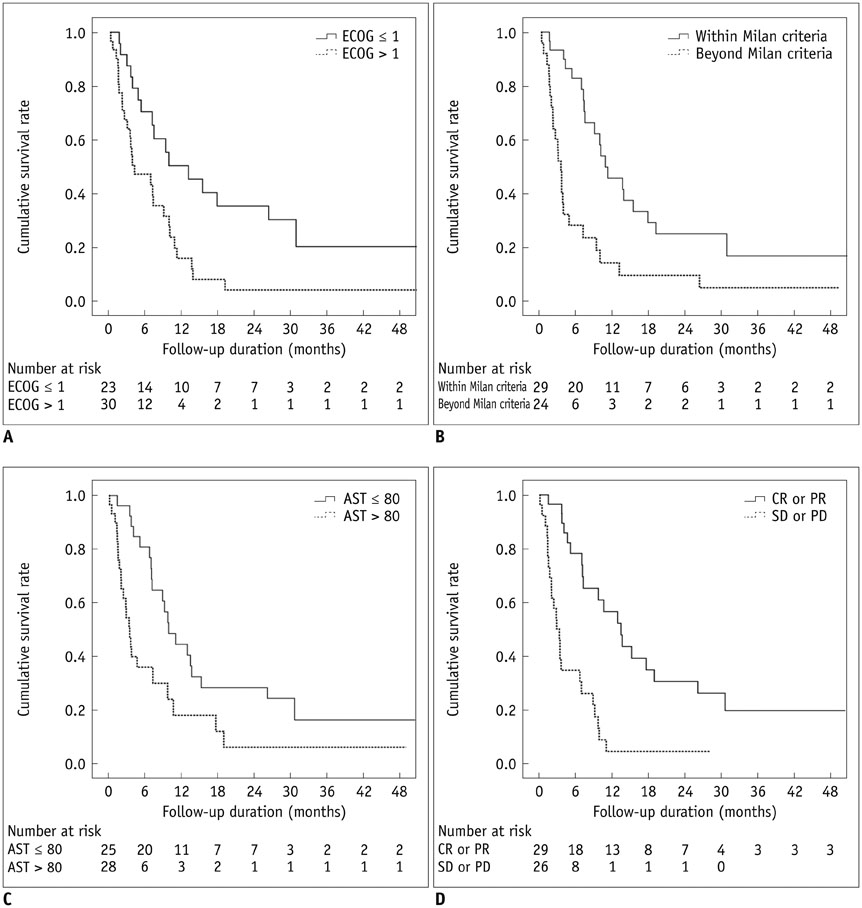
Thirty (54.5%) patients were within the Milan criteria, and 25 (45.5%) were beyond. The mortality of study subjects at 30 days was 5.5%. Major complications were observed in five (9.1%) patients who were all beyond the Milan criteria: two hepatic failures, one hepatic encephalopathy, and two CTCAE grade 3 increases in aspartate aminotransferase/alanine aminotransferase abnormality. The mean length of hospitalization was 6.3 ± 8.3 days (standard deviation), and 18 (32.7%) patients were discharged on the next day after chemoembolization. The tumor responses of the patients who met the Milan criteria were significantly higher (p = 0.014) than those of the patients who did not. The overall median survival was 7.1 months (95% confidence interval: 4.4-9.8 months).
CONCLUSION
Even in patients with Child-Pugh class C, chemoembolization can be performed safely with a selective technique in selected cases with a small tumor burden.
MeSH Terms
-
Adult
Aged
Alanine Transaminase/metabolism
Aspartate Aminotransferases/metabolism
Carcinoma, Hepatocellular/mortality/*pathology/therapy
Chemoembolization, Therapeutic/adverse effects
Female
Hepatic Encephalopathy/etiology
Humans
Length of Stay
Liver Neoplasms/mortality/*pathology/therapy
Liver Transplantation
Male
Middle Aged
Proportional Hazards Models
Severity of Illness Index
Survival Rate
Treatment Outcome
Tumor Burden
Alanine Transaminase
Aspartate Aminotransferases
Figure
Cited by 1 articles
-
Comparison of the Efficacy and Prognostic Factors of Transarterial Chemoembolization Plus Microwave Ablation versus Transarterial Chemoembolization Alone in Patients with a Large Solitary or Multinodular Hepatocellular Carcinomas
Lin Zheng, Hai-Liang Li, Chen-Yang Guo, Su-Xia Luo
Korean J Radiol. 2018;19(2):237-246. doi: 10.3348/kjr.2018.19.2.237.
Reference
-
1. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010; 30:61–74.2. Hwang S, Lee SG, Belghiti J. Liver transplantation for HCC: its role: Eastern and Western perspectives. J Hepatobiliary Pancreat Sci. 2010; 17:443–448.3. Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002; 359:1734–1739.4. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003; 37:429–442.5. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004; 127:5 Suppl 1. S179–S188.6. Eiber M, Holzapfel K, Ganter C, Epple K, Metz S, Geinitz H, et al. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: technical feasibility and assessment of lesion conspicuity in DWI. J Magn Reson Imaging. 2011; 33:1160–1170.7. Miyayama S, Matsui O, Yamashiro M, Ryu Y, Kaito K, Ozaki K, et al. Ultraselective transcatheter arterial chemoembolization with a 2-f tip microcatheter for small hepatocellular carcinomas: relationship between local tumor recurrence and visualization of the portal vein with iodized oil. J Vasc Interv Radiol. 2007; 18:365–376.8. Kim HC, Chung JW, Jae HJ, Yoon JH, Lee JH, Kim YJ, et al. Caudate lobe hepatocellular carcinoma treated with selective chemoembolization. Radiology. 2010; 257:278–287.9. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022.10. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699.11. National Cancer Institute. Common terminology criteria for adverse events (CTCAE). version 4.03. Accessed June 14, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/.12. Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001; 12:321–326.13. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.14. Brown DB, Nikolic B, Covey AM, Nutting CW, Saad WE, Salem R, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2012; 23:287–294.15. Kothary N, Weintraub JL, Susman J, Rundback JH. Transarterial chemoembolization for primary hepatocellular carcinoma in patients at high risk. J Vasc Interv Radiol. 2007; 18:1517–1526. quiz 152716. Kiely JM, Rilling WS, Touzios JG, Hieb RA, Franco J, Saeian K, et al. Chemoembolization in patients at high risk: results and complications. J Vasc Interv Radiol. 2006; 17:47–53.17. Dhanasekaran R, Khanna V, Kooby DA, Spivey JR, Parekh S, Knechtle SJ, et al. The effectiveness of locoregional therapies versus supportive care in maintaining survival within the Milan criteria in patients with hepatocellular carcinoma. J Vasc Interv Radiol. 2010; 21:1197–1204. quiz 120418. Caturelli E, Siena DA, Fusilli S, Villani MR, Schiavone G, Nardella M, et al. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of damage to nontumorous liver tissue-long-term prospective study. Radiology. 2000; 215:123–128.19. Miyoshi S, Minami Y, Kawata S, Imai Y, Saitoh R, Noda S, et al. Changes in hepatic functional reserve after transcatheter embolization of hepatocellular carcinoma. Assessment by maximal removal rate of indocyanine green. J Hepatol. 1988; 6:332–336.20. Khan KN, Nakata K, Kusumoto Y, Shima M, Ishii N, Koji T, et al. Evaluation of nontumorous tissue damage by transcatheter arterial embolization for hepatocellular carcinoma. Cancer Res. 1991; 51:5667–5671.21. Miyayama S, Mitsui T, Zen Y, Sudo Y, Yamashiro M, Okuda M, et al. Histopathological findings after ultraselective transcatheter arterial chemoembolization for hepatocellular carcinoma. Hepatol Res. 2009; 39:374–381.22. Miyayama S, Yamashiro M, Okuda M, Yoshie Y, Sugimori N, Igarashi S, et al. Usefulness of cone-beam computed tomography during ultraselective transcatheter arterial chemoembolization for small hepatocellular carcinomas that cannot be demonstrated on angiography. Cardiovasc Intervent Radiol. 2009; 32:255–264.23. Choi WS, Kim HC, Hur S, Choi JW, Lee JH, Yu SJ, et al. Role of C-arm CT in identifying caudate arteries supplying hepatocellular carcinoma. J Vasc Interv Radiol. 2014; 25:1380–1388.24. Lee IJ, Chung JW, Yin YH, Kim HC, Kim YI, Jae HJ, et al. Cone-beam CT hepatic arteriography in chemoembolization for hepatocellular carcinoma: angiographic image quality and its determining factors. J Vasc Interv Radiol. 2014; 25:1369–1379. quiz 1379–quiz 1379.e1.25. Bismuth H, Morino M, Sherlock D, Castaing D, Miglietta C, Cauquil P, et al. Primary treatment of hepatocellular carcinoma by arterial chemoembolization. Am J Surg. 1992; 163:387–394.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transcatheter Arterial Chemoembolization for Infiltrative Hepatocellular Carcinoma: Clinical Safety and Efficacy and Factors Influencing Patient Survival
- Risk Factors for Liver Function Deterioration after Transarterial Chemoembolization Refractoriness in Child-Pugh Class A Hepatocellular Carcinoma Patients
- The effect of Transarterial Chemoembolization(TAE) on Lung metastasis of Hepatocellular Carcinoma
- Outcome of Hepatic Resection for Hepatocellular Carcinoma within the Milan Criteria in Child-Pugh Class A Patients
- The effect of nucleos(t)ide analogues on clinical outcomes of patients treated with transarterial chemoembolization and radiofrequency ablation for hepatitis B virus-related hepatocellular carcinoma