J Korean Ophthalmol Soc.
2007 Aug;48(8):1134-1142.
Identification of P2Y11 Receptor Expressed in Human Retinoblastoma Cells
- Affiliations
-
- 1Shimmian Oculoplastic Center, Seoul, Korea.
- 2Department of Basic Nursing Science Keimyung University College of Nursing, Daegu, Korea.
- 3Department of Ophthalmology, Yonsei University Wonju College of Medicine, Wonju, Korea. jhlee@wonju.yonsei.ac.kr
- 4Department of Physiology and Institute of Basic Medical Science, Wonju, Korea.
Abstract
-
PURPOSE: The present study aimed to identify the characteristics and physiological function of the P2Y11 receptor, a receptor likely expressed in human retinoblastoma cells.
METHODS
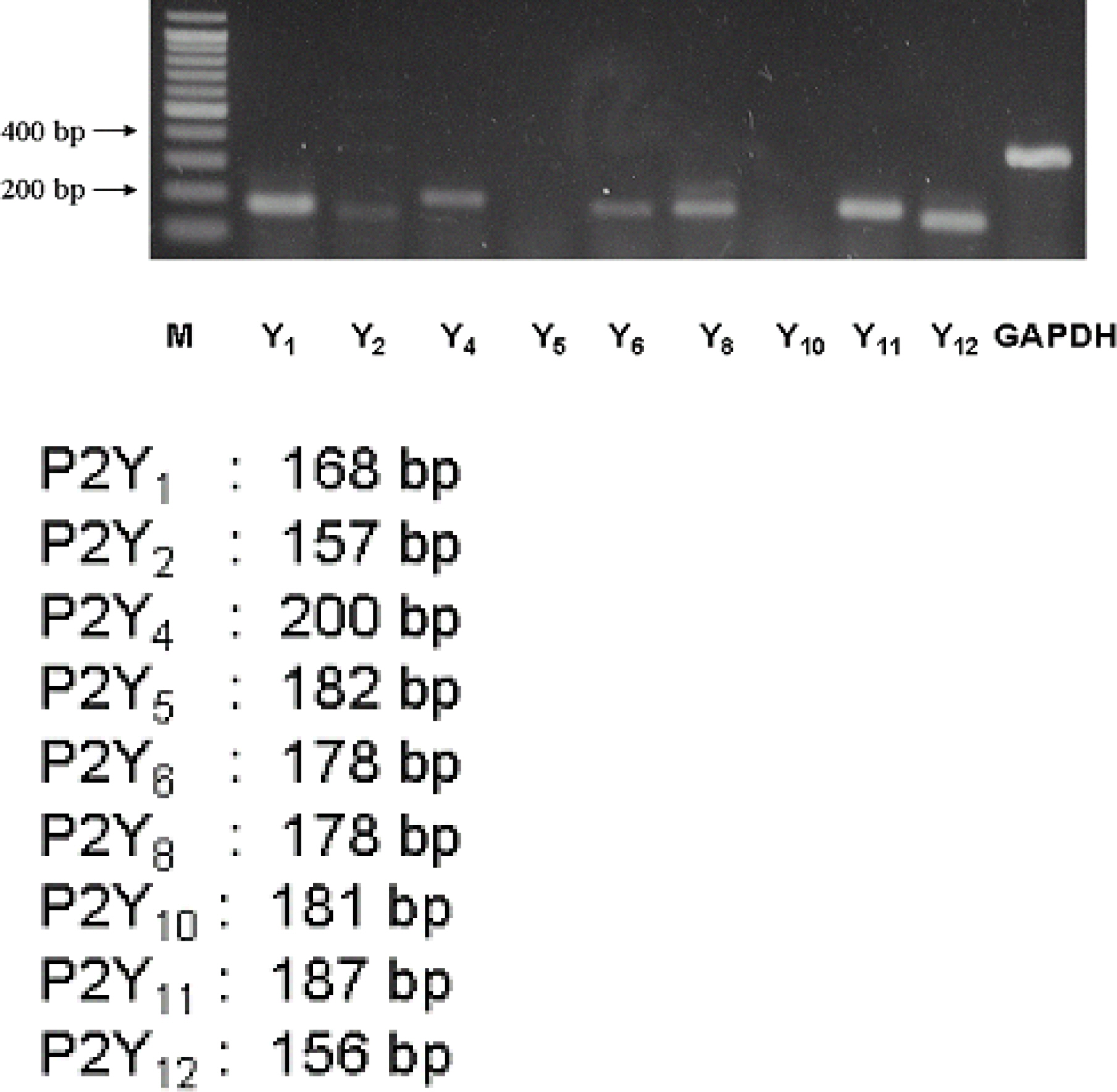
We measured possible P2Y11 signaling in WERI-Rb-1 cells using a Ca2+ imaging technique and RT-PCR.
RESULTS
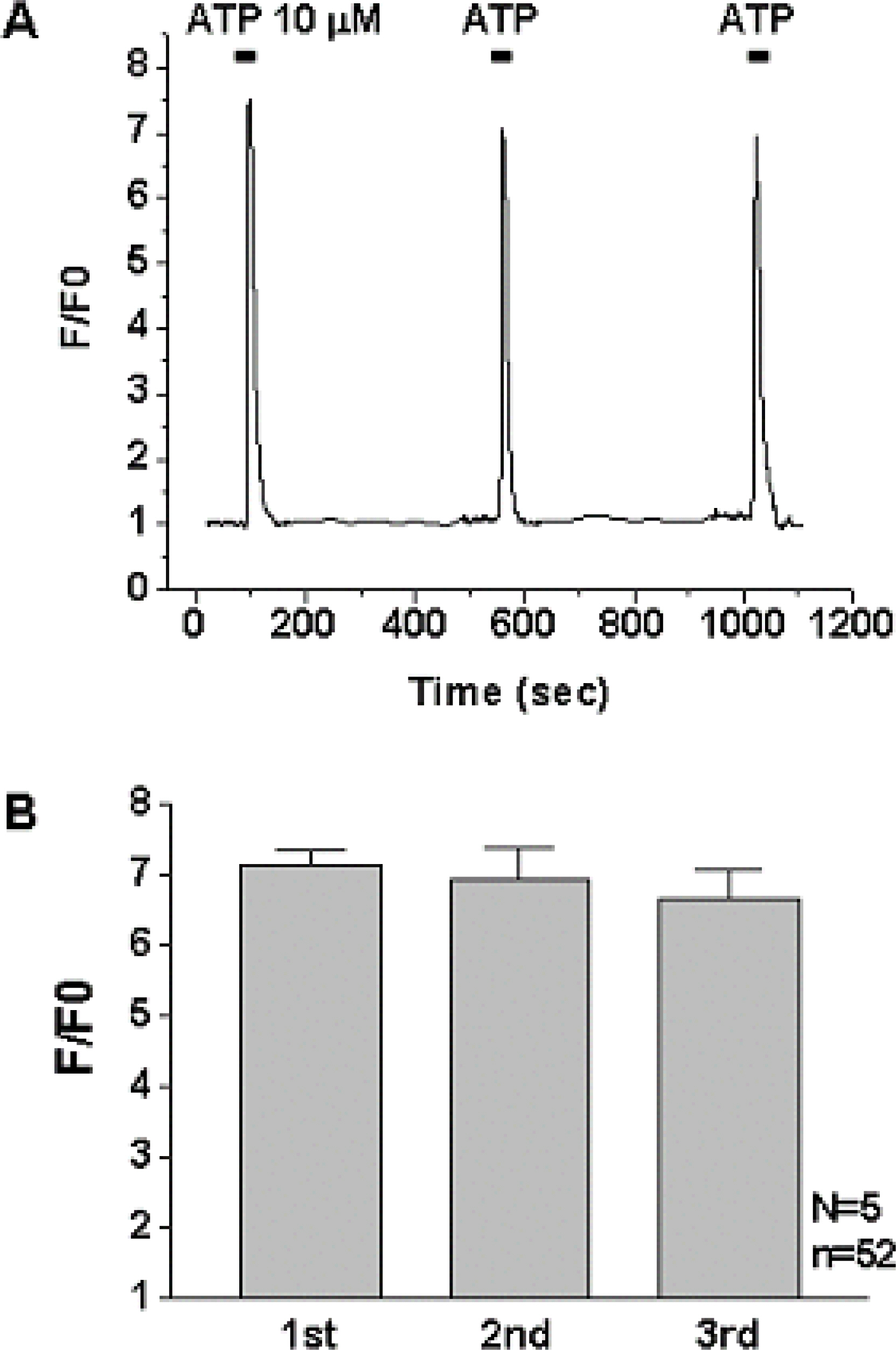
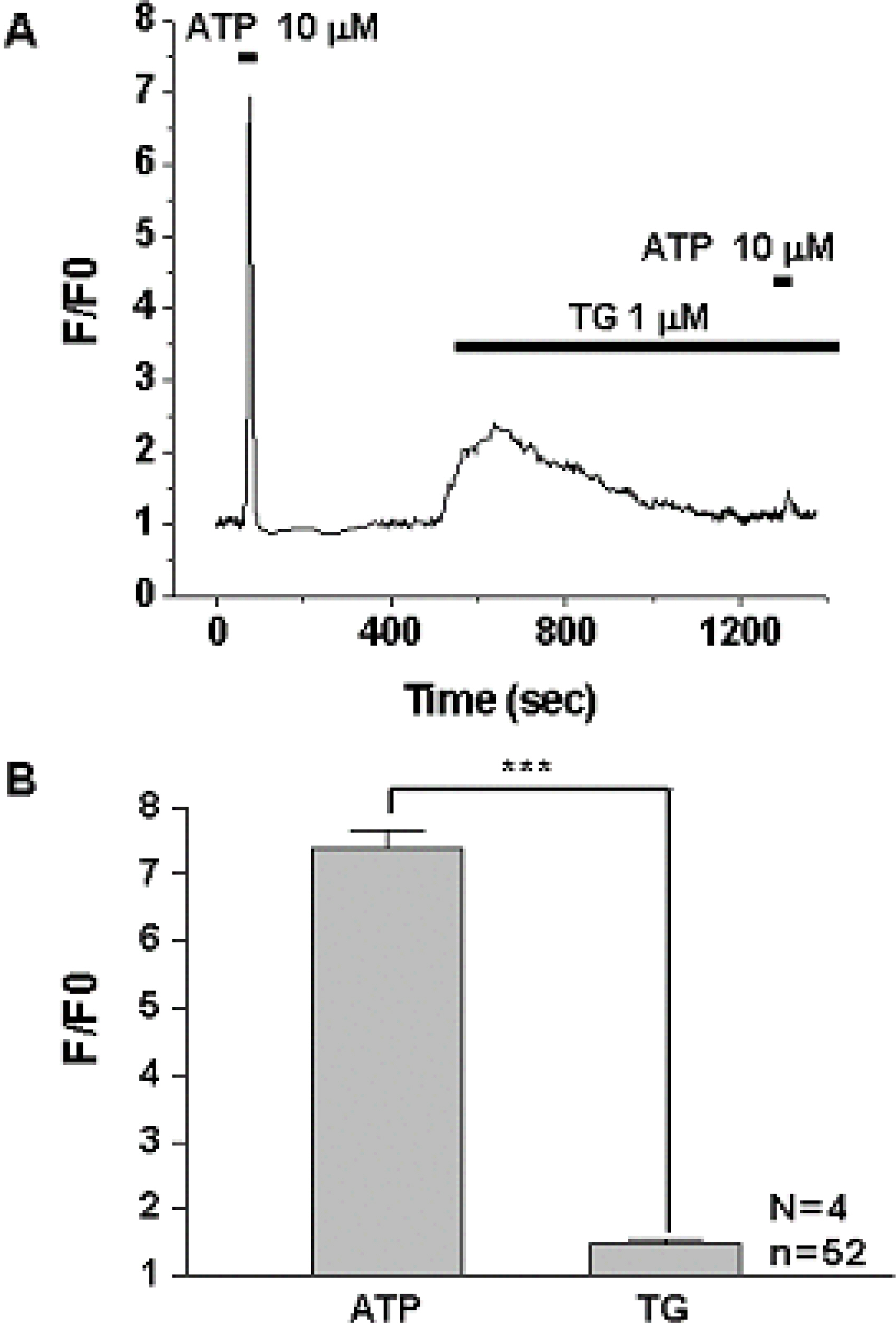
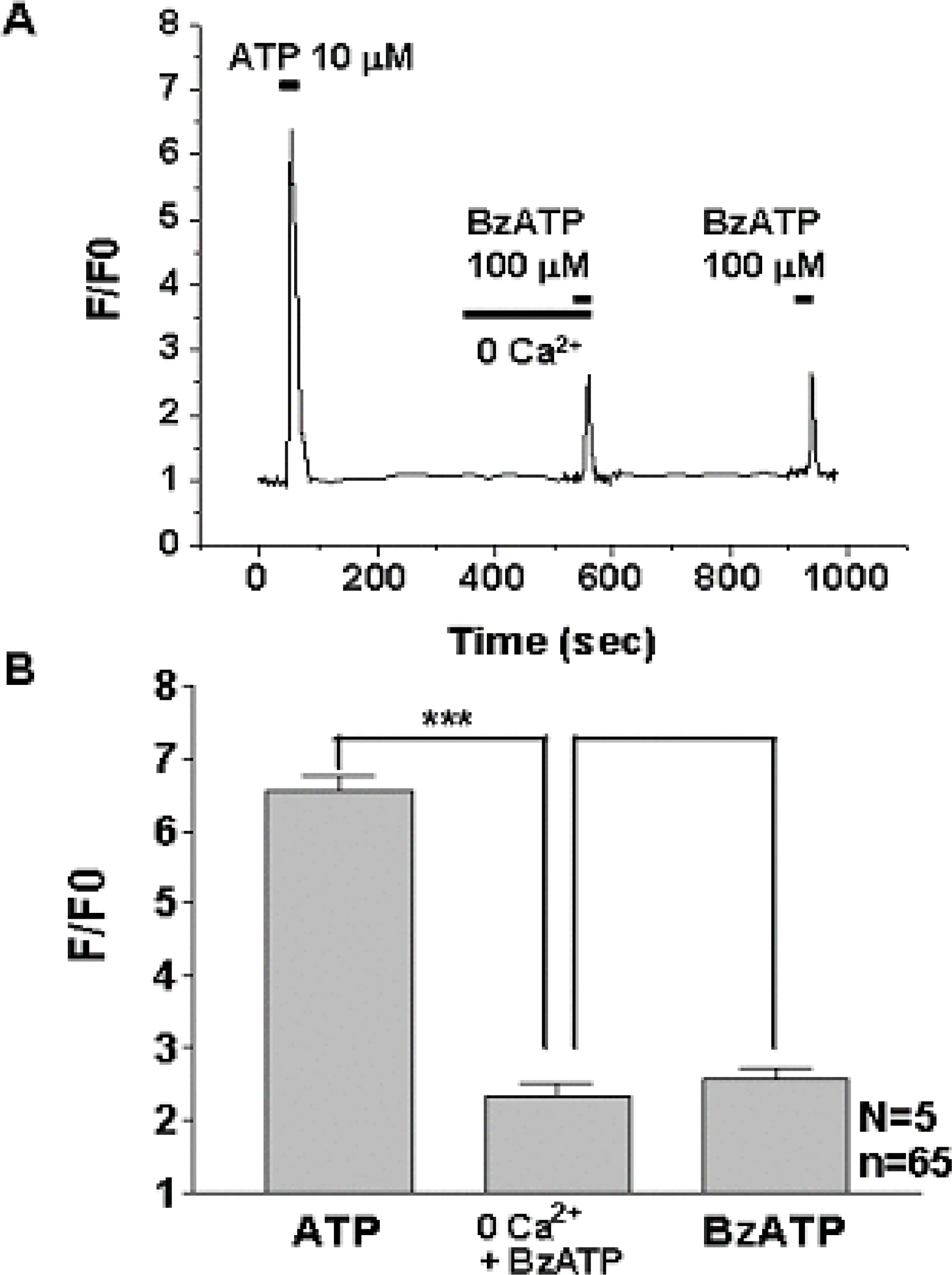
1) 10 micro M ATP elicited a strong but transient increase in Ca2+ in the WERI-Rb-1 cells, and this Ca2+ rise was well maintained after external Ca2+-depletion. 2) ATP-induced Ca2+ response arose entirely through Ca2+ mobilization. 3) P2Y11 agonist (BzATP, 100 micro M) increased Ca2+ by 31.2+/-3.7 % of ATP effect. 4) mRNA for P2Y11 subtype was identified using RT-PCR.
CONCLUSIONS
P2Y11 purinergic activation can increase the intracellular calcium level through calcium mobilization in undifferentiated retinoblastoma cells, which may play an important role in cell proliferation, differentiation, and even pathologic processes.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Burnstock G. Overview: purinergic mechanism. Ann NY Acad Sci. 1990; 603:1–17.2. Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998; 50:413–92.3. Fries JE, Wheeler-Schilling TH, Kohler K, Guenther E. Distribution of metabotropic P2Y receptors in the rat retina: a single cell RT-PCR study. Mol Brain Res. 2004; 130:1–6.4. Sak K, Webb TE. A retrospective of recombinant P2Y receptor subtypes and their pharmacology. Arch Biochem Biophys. 2002; 397:131–6.
Article5. Abbracchio MP, Boeynaems JM, Barnard EA, et al. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003; 24:52–5.
Article6. Fries JE, Wheeler-Schilling TH, Guenther E, Kohler K. Expression of P2Y1, P2Y2, P2Y4, and P2Y6 receptor subtypes in the rat retina. Invest Ophthalmol Vis Sci. 2004; 45:3410–4.
Article7. Cha SH, Hahn TW, Sekine T, et al. Purinoceptor-mediated calcium mobilization and cellular proliferation in cultured bovine corneal endothelial cells. Jpn J Pharmacol. 2000; 82:181–7.
Article8. Abbracchio MP, Burnstock G. Purinergic signaling: Pathophysiological roles. Jpn J pharmacol. 1998; 78:113–45.9. Jumblatt JE, Jumblatt MM. Regulation of ocular mucin secretion by P2Y2 nucleotide receptors in rabbit and human conjunctiva. Exp Eye Res. 1998; 67:341–6.10. Pintor J. Purinergic signaling in the eye. In : Burnstock G, Sillito AM, editors. The autonomic nervous system. 4th ed.London: Harwood Academic Publisher;1998. chap. 20-21.11. Kimura K, Nishimura T, Satoh Y. Effect of ATP and its analogues on [Ca2+]i dynamics in the rabbit corneal epithelium. Arch Histol Cytol. 1999; 62:129–38.12. Murakami T, Fujihara T, Nakamura M, Nakata K. P2Y (2) receptor stimulation increases tear fluid secretion in rabbits. Curr Eye Res. 2000; 21:782–7.13. Shiue MH, Kulkarni AA, Gukasvan HJ, et al. Pharmacological modulation of fluid secretion in the pigmented rabbit conjunctiva. Life Sci. 2000; 66:105–11.
Article14. Cowlen MS, Zhang VZ, Warnock L, et al. Localization of ocular P2Y2 receptor gene expression by in situ hybridization. Exp Eye Res. 2003; 77:77–84.
Article15. Srinivas SP, Yeh JC, Ong A, Bonanno JA. Ca2+ mobilization in bovine corneal endothelial cells by P2 purinergic recptors. Curr Eye Res. 1998; 17:994–1004.16. Yang H, Reinach PS, Koniarek JP, et al. Fluid transport by cultured corneal epithelial cell layers. Br J Ophthalmol. 2000; 84:199–204.
Article17. Li Y, Kuang K, Yerxa B, et al. Rabbit conjunctival epithelium transports fluid, and P2Y2 receptor agonists stimulate Cl- and fluid secretion. Am J Physiol Cell Physiol. 2001; 281:595–602.18. Wax M, Sanghavi DM, Lee CH, Kapadia M. Purinergic receptors in ocular ciliary epithelial cells. Exp Eye Res. 1993; 57:89–95.
Article19. Shahidullah M, Wilson WS. Mobilisation of intracellular calcium by P2Y2 receptors in cultured, non-transformed bovine ciliary epithelial cells. Curr Eye Res. 1997; 16:1006–16.
Article20. Liu Y, Wakakura M. P1-/P2-purinergic receptors on cultured rabbit retinal muller cells. Jpn J Ophthalmol. 1998; 42:33–40.21. Bringmann A, Pannicke T, Weick M, et al. Activation of P2Y receptors stimulates potassium and cation currents in acutely isolated human Muller (glial) cells. Glia. 2002; 37:139–52.22. Sullivan DM, Erb L, Anglade E, et al. Identification and characterization of P2Y2 nucleotide receptors in human retinal pigment epithelial cells. J Neurosci Res. 1997; 49:43–52.
Article23. Sugamoto Y, Hirai K, Tokoro T. P2Y2 receptor elevates intracellular calcium concentration in rabbit eye suprachoroid. J Med Dent Sci. 1999; 46:83–92.24. Collison DJ, Duncan G. Regional differences in functional receptor distribution and calcium mobilization in the intact human lens. Invest Ophthalmol Vis Sci. 2001; 42:2355–63.25. James G, Butt AM. Changes in P2Y and P2X purinoceptors in reactive glia following axonal degeneration in the rat optic nerve. Neurosci Lett. 2001; 312:33–6.
Article26. Li Y, Holtzclaw LA, Russell JT. Muller cell Ca2+ waves evoked by purinergic receptor agonists in slices of rat retina. J Neurophysiol. 2001; 85:986–94.27. Kyritsis AP, Tsokos M, Triche TJ, Chader GJ. Retinoblastoma -origin from a primitive neuroectodermal tumer cell? Nature. 1984; 307:471–3.28. Pearson R, Catsicas M, Becker D, Mobbs P. Purinergic and muscarinic modulation of the cell cycle and calcium signaling in the chick retinal ventricular zone. J Neurosci. 2002; 22:7569–79.
Article29. Mitchell CH. Release of ATP by a human retinal pigment epithelial cell line: potential for autocrine stimulation through subretinal space. J Physiol. 2001; 534:193–202.
Article30. McFall RC, Sery TW, Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977; 37:1003–10.31. Kim DR, Cha SK, Kong ID, et al. Characteristics of nicotinic receptor expressed in human retinoblastoma. J Korean Opthalmol Soc. 2005; 46:1060–7.32. Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Annal Biochem. 1987; 162:156–9.
Article33. Adrian K, Bernhard MK, Breitinger HG, Ogilve A. Expression of purinergic receptors (ionotropic P2X1-7 and metabotropic P2Y1-11) during myeloid differentiation of HL60 cells. Biochim Biophys Acta. 2000; 1492:127–38.
Article34. Sanches G, de Alencar LS, Ventura AL. ATP induces proliferation of retinal cells in culture via activation of PKC and extracellular signal-regulated cascade. Int J Dev Neurosci. 2002; 20:21–7.35. Harden TK, Boyer JL, Nicholas RA. P2-purinergic receptors: subtype- associated signaling responses and structure. Annu Rev Pharmacol Toxicol. 1995; 35:541–79.36. Schachter JB, Li Q, Boyer JL, et al. Second messenger cascade specificity and pharmacological selectivity of the human P2Y1- purinoceptor. Br J Pharmacol. 1996; 118:167–73.37. Kugelgen IV, Wetter A. Molecular pharmacology of P2Y-receptors. Naunyn- Schmiedelberg's Arch Pharmacol. 2000; 362:310–23.38. Chen ZP, Levy A, Lightman SL. Nucleotides as extracellular signalling molecules. J Neuroendocrinol. 1995; 7:83–96.
Article39. Communi D, Govaerts C, Parmentier M, Boeynaems JM. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. J Biol Chem. 1997; 272:31969–73.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characteristics of Nicotinic Receptor Expressed in Human Retinoblastoma
- UTP and ATPgammaS Induce Mucin Secretion via Ca2+ Dependant Pathways in Human Nasal Epithelial Cells
- Effect of Uridine 5'-Triphosphate on Mucin Secretion in Human Middle Ear Epithelial Cells
- Enzyme Histochemical Study of Retinoblastoma
- The 13q14 Deletion in Retinoblastoma