J Korean Rheum Assoc.
2010 Sep;17(3):238-245.
IL-15 Induced an Increased SDF-1 Expression in the Synovial Fibroblasts of Patients with Rheumatoid Arthritis
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Pusan National University, Busan, Korea. ksimd@pusan.ac.kr
- 2The Rheumatism Resarch Center, Catholic Research Institute of Medical Science, The Catholic University of Korea, Seoul, Korea.
Abstract
OBJECTIVE
Interleukin-15 (IL-15) recruits and activates synovial T cells, and IL-15 plays an important role in amplifying and perpetuating inflammation in the pathogenesis of rheumatoid arthritis (RA). Stromal cell-derived factor-1 (SDF-1) is a potent chemoattractant for memory T cells in the inflamed RA synovium. This study investigated the effect of IL-15 on SDF-1 production in RA fibroblast-like synoviocytes (FLS).
METHODS
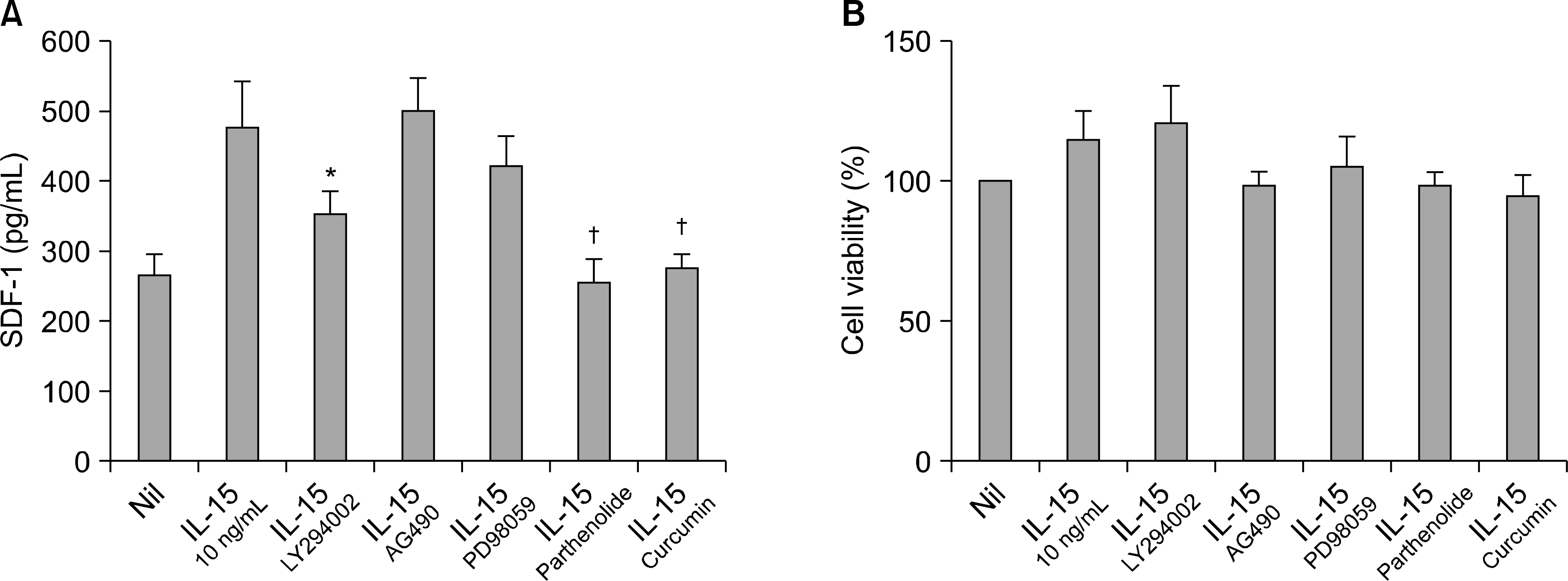
The expressions of IL-15 and SDF-1 were determined from the synovium of patients with RA and osteoarthritis (OA) by performing immunohistochemistry. The expressions of SDF-1 was measured from the RA FLS that were cultured with IL-15 and IL-17 by real-time RT-PCR and ELISA. The SDF-1 expression was also measured, via ELISA, from the RA FLS stimulated by IL-15 together with the inhibitors of such intracellular signal molecules as phosphatidylinositol 3-kinase (PI 3-kinase, LY294002), STAT3 (AG490), MAP Kinase (PD98059), NF-kappaB (parthenolide) and activator protein 1 (AP-1, curcumin).
RESULTS
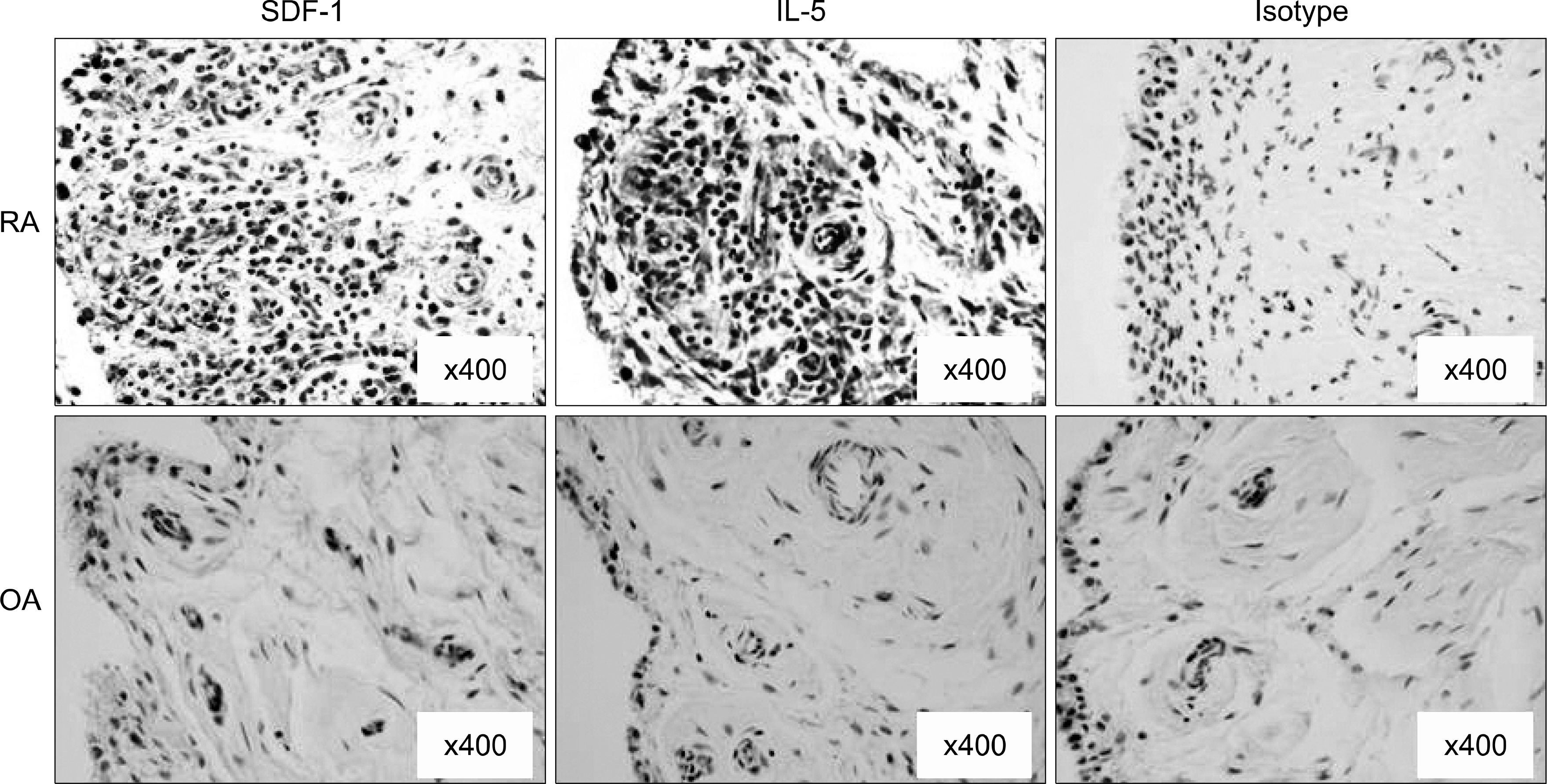
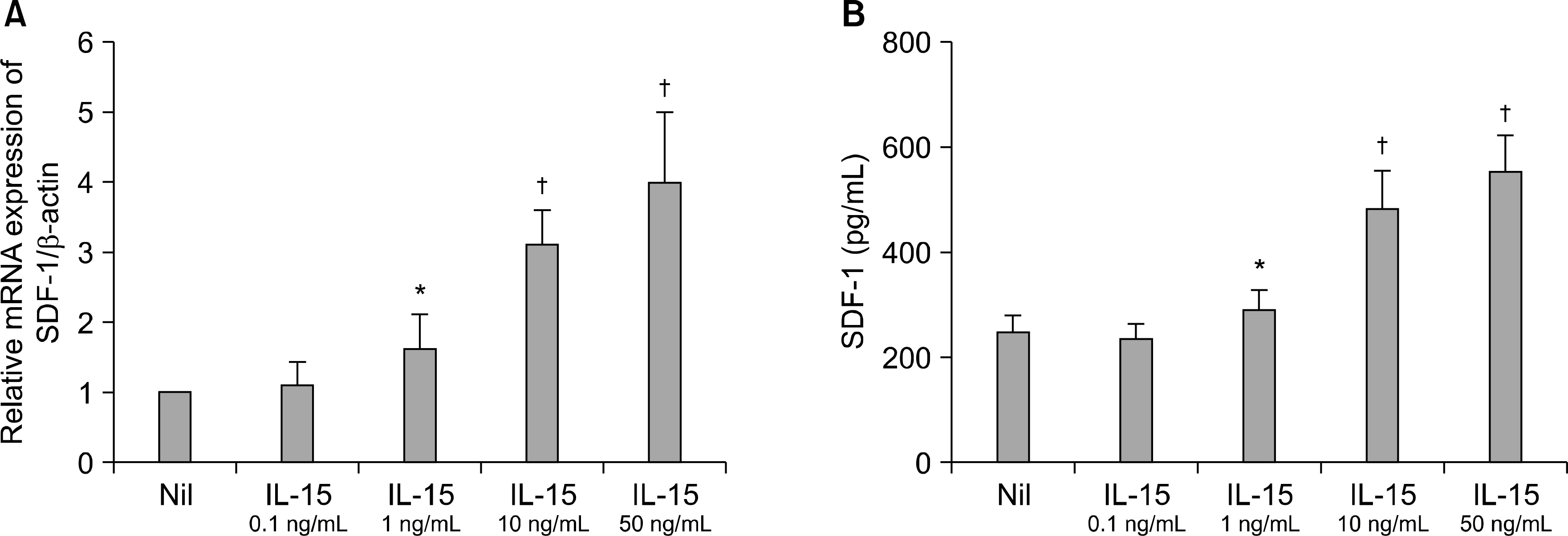
IL-15 and SDF-1 were mainly expressed in the RA synovium compared to that of the OA synovium. IL-15 increased the SDF-1 expressions and it, and had an additive effect with IL-17 on the SDF-1 expressions in the cultured RA FLS. The IL-15 induced increase of the SDF-1 expression in the cultured RA FLS was blocked by the inhibitors of PI 3-kinase, NF-kappaB and AP-1.
CONCLUSION
The SDF-1 expression was increased in the RA synovium and it was up-regulated by IL-15 in the RA FLS through the PI 3-kinase, NF-kappaB, and AP-1 pathways. These results imply that the IL-15 induced increase of the SDF-1 expressions may be involved in the immunopathogenesis of RA.
Keyword
MeSH Terms
-
Arthritis, Rheumatoid
Enzyme-Linked Immunosorbent Assay
Fibroblasts
Humans
Immunohistochemistry
Inflammation
Interleukin-15
Interleukin-17
Memory
NF-kappa B
Osteoarthritis
Phosphatidylinositol 3-Kinase
Phosphatidylinositol 3-Kinases
Phosphotransferases
Synovial Membrane
T-Lymphocytes
Transcription Factor AP-1
Interleukin-15
Interleukin-17
NF-kappa B
Phosphatidylinositol 3-Kinase
Phosphatidylinositol 3-Kinases
Phosphotransferases
Transcription Factor AP-1
Figure
Reference
-
1). MuUller-Ladner U., Pap T., Gay RE., Neidhart M., Gay S. Mechanisms of disease: the molecular and cellular basis of joint destruction in rheumatoid arthritis. Nat Clin Pract Rheumatol. 2005. 1:102–10.2). Mittal GA., Joshi VR., Deshpande A. Stromal cell-derived factor-1 alpha in rheumatoid arthritis. Rheumatology (Oxford). 2003. 42:915–6.3). Kanbe K., Takagishi K., Chen Q. Stimulation of matrix metalloprotease 3 release from human chondrocytes by the interaction of stromal cell-derived factor 1 and CXC chemokine receptor 4. Arthritis Rheum. 2002. 46:130–7.
Article4). Grassi F., Cristino S., Toneguzzi S., Piacentini A., Facchini A., Lisignoli G. CXCL12 chemokine up-regulates bone resorption and MMP-9 release by human osteoclasts: CXCL12 levels are increased in synovial and bone tissue of rheumatoid arthritis patients. J Cell Physiol. 2004. 199:244–51.
Article5). Pablos JL., Santiago B., Galindo M., Torres C., Brehmer MT., Blanco FJ, et al. Synoviocyte-derived CXCL12 is displayed on endothelium and induces angiogenesis in rheumatoid arthritis. J Immunol. 2003. 170:2147–52.
Article6). Nanki T., Hayashida K., El-Gabalawy HS., Suson S., Shi K., Girschick HJ, et al. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J Immunol. 2000. 165:6590–8.7). De Klerck B., Geboes L., Hatse S., Kelchtermans H., Meyvis Y., Vermeire K, et al. Pro-inflammatory properties of stromal cellderived factor-1 (CXCL12) in collagen-induced arthritis. Arthritis Res Ther. 2005. 7:R1208–20.8). Tamamura H., Fujisawa M., Hiramatsu K., Mizumoto M., Nakashima H., Yamamoto N, et al. Identification of a CXCR4 antagonist, a T140 analog, as an anti-rheumatoid arthritis agent. FEBS Lett. 2004. 569:99–104.
Article9). McInnes IB., al-Mughales J., Field M., Leung BP., Huang FP., Dixon R, et al. The role of interleukin-15 in T-cell migration and activation in rheumatoid arthritis. Nat Med. 1996. 2:175–82.
Article10). Thurkow EW., van der Heijden IM., Breedveld FC., Smeets TJ., Daha MR., Kluin PM, et al. Increased expression of IL-15 in the synovium of patients with rheumatoid arthritis compared with patients with Yersinia-induced arthritis and osteoarthritis. J Pathol. 1997. 181:444–50.11). Oppenheimer-Marks N., Brezinschek RI., Mohamadzadeh M., Vita R., Lipsky PE. Interleukin 15 is produced by endothelial cells and increases the transendothelial migration of T cells in vitro and in the SCID mouse-human rheumatoid arthritis model in vivo. J Clin Invest. 1998. 101:1261–72.
Article12). Miranda-CaruAs ME., Balsa A., Benito-Miguel M., PeArez de Ayala C., MartiAn-Mola E. IL-15 and the initiation of cell contact-dependent synovial fibroblast-T lymphocyte cross-talk in rheumatoid arthritis: effect of methotrexate. J Immunol. 2004. 173:1463–76.
Article13). Cho ML., Yoon CH., Hwang SY., Park MK., Min SY., Lee SH, et al. Effector function of type II collagen-stimulated T cells from rheumatoid arthritis patients: cross-talk between T cells and synovial fibroblasts. Arthritis Rheum. 2004. 50:776–84.
Article14). Ruchatz H., Leung BP., Wei XQ., McInnes IB., Liew FY. Soluble IL-15 receptor alpha-chain administration prevents murine collagen-induced arthritis: a role for IL-15 in development of antigen-induced immunopathology. J Immunol. 1998. 160:5654–60.15). Ferrari-Lacraz S., Zanelli E., Neuberg M., Donskoy E., Kim YS., Zheng XX, et al. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol. 2004. 173:5818–26.
Article16). Baslund B., Tvede N., Danneskiold-Samsoe B., Larsson P., Panayi G., Petersen J, et al. Targeting interleukin-15 in patients with rheumatoid arthritis: a proof-of-concept study. Arthritis Rheum. 2005. 52:2686–92.
Article17). Murdoch C. CXCR4: chemokine receptor extraordinaire. Immunol Rev. 2000. 177:175–84.
Article18). Mbemba E., Benjouad A., Saffar L., Gattegno L. Glycans and proteoglycans are involved in the interactions of human immunodeficiency virus type 1 envelope glycoprotein and of SDF-1aL with membrane ligands of CD4+ CXCR4+ cells. Virology. 1999. 265:354–64.19). Burger JA., Kipps TJ. CXCR4: a key receptor in the cross talk between tumor cells and their microenvironment. Blood. 2006. 107:1761–7.20). Dubois-Laforgue D., Hendel H., Caillat-Zucman S., Zagury JF., Winkler C., Boitard C, et al. A common stromal cell-derived factor-1 chemokine gene variant is associated with the early onset of type 1 diabetes. Diabetes. 2001. 50:1211–3.
Article21). Hitchon C., Wong K., Ma G., Reed J., Lyttle D., El-Gabalawy H. Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum. 2002. 46:2587–97.
Article22). Kim KW., Cho ML., Kim HR., Ju JH., Park MK., Oh HJ, et al. Up-regulation of stromal cell-derived factor 1 (CXCL12) production in rheumatoid synovial fibroblasts through interactions with T lymphocytes: role of interleukin-17 and CD40L-CD40 interaction. Arthritis Rheum. 2007. 56:1076–86.
Article23). Groh V., Bruhl A., El-Gabalawy H., Nelson JL., Spies T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc Natl Acad Sci USA. 2003. 100:9452–57.
Article24). Ernestam S., af Klint E., Catrina AI., Sundberg E., EngstroUm M., Klareskog L, et al. Synovial expression of IL-15 in rheumatoid arthritis is not influenced by blockade of tumour necrosis factor. Arthritis Res Ther. 2006. 8:R18.25). Giri JG., Kumaki S., Ahdieh M., Friend DJ., Loomis A., Shanebeck K, et al. Identification and cloning of a novel IL-15 binding protein that is structurally related to the alpha chain of the IL-2 receptor. EMBO J. 1995. 14:3654–63.
Article26). Waldmann T., Tagaya Y., Bamford R. Interleukin-2, interleukin-15, and their receptors. Int Rev Immunol. 1998. 16:205–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hypoxia/reoxygenation Regulates Expression of Tumor Suppressor PTEN in Rheumatoid Synovial Fibroblasts
- Effects of TGF-beta, GM-CSF, and PDGF on Proliferation and Expression of Cytokine and Metalloproteinase Genes in Rheumatoid Synovial Cells
- Macrophage Migration Inhibitory Factor (MIF) Induced Stromal Cell-derived Factor 1 (SDF-1) Production Via Nuclear Factor KappaB (NF-kappaB) Signaling in Rheumatoid Arthritis Fibroblast Like Synoviocytes (RA-FLS)
- Selective Susceptibility of Synovial Fibroblasts Isolated from Patients with Rheumatoid Arthritis to TRAIL -induced Cell Death
- Synovial Chondromatosis in Knee Masquerading as Tuberculosis Arthritis