J Periodontal Implant Sci.
2013 Dec;43(6):291-300.
Dissolution behavior and early bone apposition of calcium phosphate-coated machined implants
- Affiliations
-
- 1Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 2Institute of Physics & Applied Physics, Atomic-Scale Surface Science Research Center, Yonsei University, Seoul, Korea.
Abstract
- PURPOSE
Calcium phosphate (CaP)-coated implants promote osseointegration and survival rate. The aim of this study was to (1) analyze the dissolution behavior of the residual CaP particles of removed implants and (2) evaluate bone apposition of CaP-coated machined surface implants at the early healing phase.
METHODS
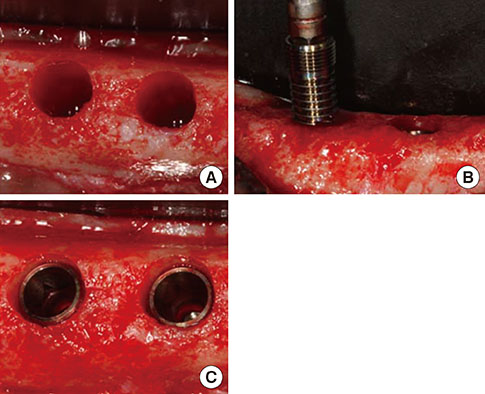
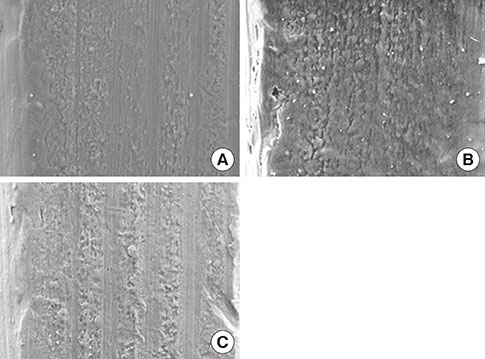
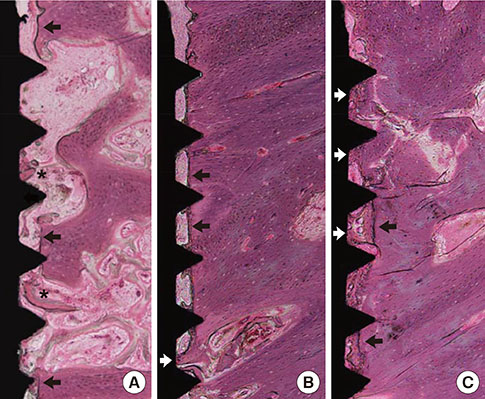
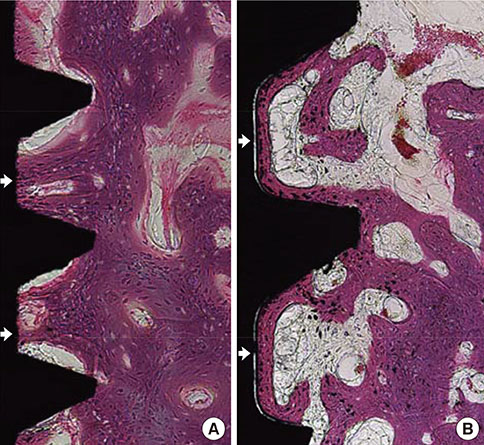
Mandibular premolars were extracted from five dogs. After eight weeks, the implants were placed according to drilling protocols: a nonmobile implant (NI) group and rotational implant (RI) group. For CaP dissolution behavior analysis, 8 implants were removed after 0, 1, 2, and 4 weeks. The surface morphology and deposition of the coatings were observed. For bone apposition analysis, block sections were obtained after 1-, 2-, and 4-week healing periods and the specimens were analyzed.
RESULTS
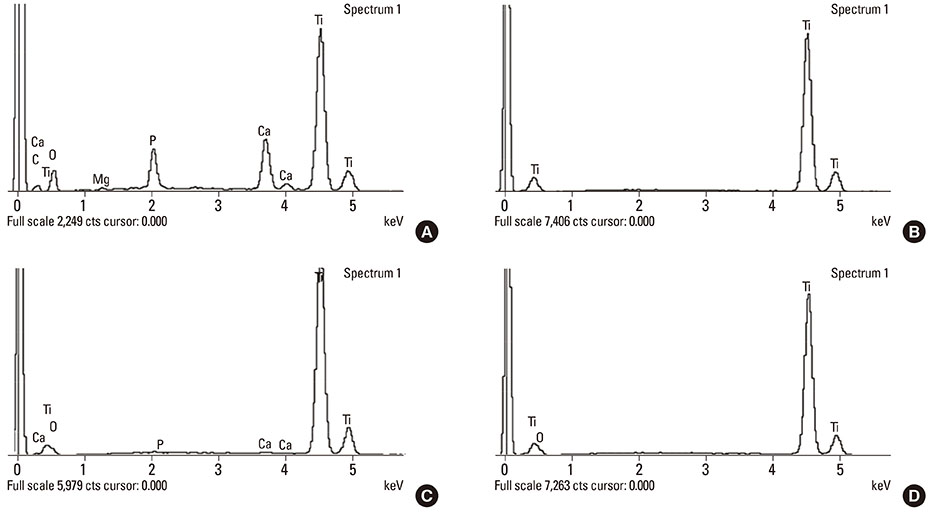
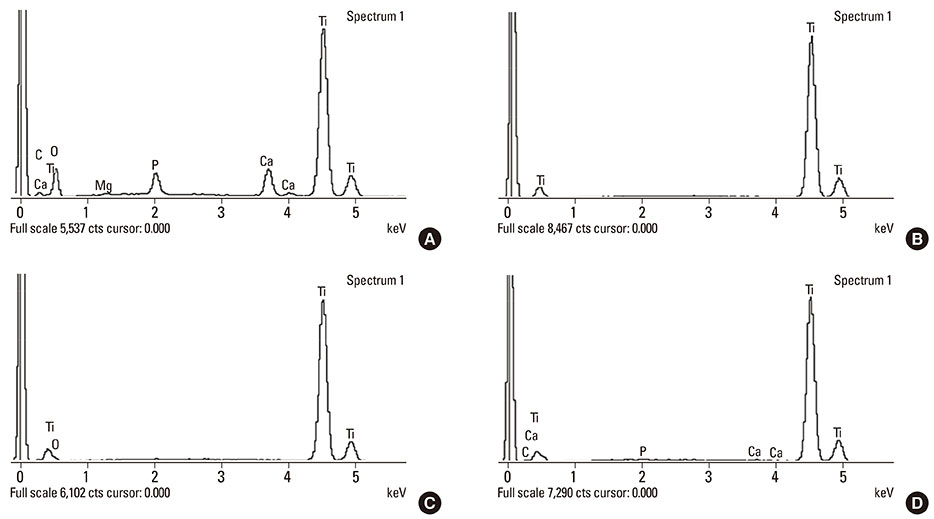
Calcium and phosphorus were detected in the implants that were removed immediately after insertion, and the other implants were composed mainly of titanium. There were no notable differences between the NI and RI groups in terms of the healing process. The bone-to-implant contact and bone density in the RI group showed a remarkable increase after 2 weeks of healing.
CONCLUSIONS
It can be speculated that the CaP coating dissolves early in the healing phase and chemically induces early bone formation regardless of the primary stability.
MeSH Terms
Figure
Reference
-
1. Tjellstrom A, Lindstrom J, Hallen O, Albrektsson T, Branemark PI. Osseointegrated titanium implants in the temporal bone: a clinical study on bone-anchored hearing aids. Am J Otol. 1981; 2:304–310.2. Cooper LF. A role for surface topography in creating and maintaining bone at titanium endosseous implants. J Prosthet Dent. 2000; 84:522–534.
Article3. Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NH. Implant surface roughness and bone healing: a systematic review. J Dent Res. 2006; 85:496–500.
Article4. Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res. 2009; 20:Suppl 4. 172–184.
Article5. Han T, Carranza FA Jr, Kenney EB. Calcium phosphate ceramics in dentistry: a review of the literature. J West Soc Periodontol Periodontal Abstr. 1984; 32:88–108.6. Davies JE. Understanding peri-implant endosseous healing. J Dent Educ. 2003; 67:932–949.
Article7. Morimoto K, Kihara A, Takeshita F, Akedo H, Suetsugu T. Differences between the bony interfaces of titanium and hydroxyapatite-alumina plasma-sprayed titanium blade implants. J Oral Implantol. 1988; 14:314–324.8. Kay JF. Calcium phosphate coatings for dental implants. Current status and future potential. Dent Clin North Am. 1992; 36:1–18.9. McGlumphy EA, Peterson LJ, Larsen PE, Jeffcoat MK. Prospective study of 429 hydroxyapatite-coated cylindric omniloc implants placed in 121 patients. Int J Oral Maxillofac Implants. 2003; 18:82–92.10. Biesbrock AR, Edgerton M. Evaluation of the clinical predictability of hydroxyapatite-coated endosseous dental implants: a review of the literature. Int J Oral Maxillofac Implants. 1995; 10:712–720.11. Liao H, Fartash B, Li J. Stability of hydroxyapatite-coatings on titanium oral implants (IMZ): 2 retrieved cases. Clin Oral Implants Res. 1997; 8:68–72.
Article12. Hanisch O, Cortella CA, Boskovic MM, James RA, Slots J, Wikesjo UM. Experimental peri-implant tissue breakdown around hydroxyapatite-coated implants. J Periodontol. 1997; 68:59–66.13. Klein CP, Patka P, van der Lubbe HB, Wolke JG, de Groot K. Plasma-sprayed coatings of tetracalciumphosphate, hydroxyl-apatite, and alpha-TCP on titanium alloy: an interface study. J Biomed Mater Res. 1991; 25:53–65.
Article14. Hayashi K, Inadome T, Mashima T, Sugioka Y. Comparison of bone-implant interface shear strength of solid hydroxyapatite and hydroxyapatite-coated titanium implants. J Biomed Mater Res. 1993; 27:557–563.
Article15. Albrektsson T. Hydroxyapatite-coated implants: a case against their use. J Oral Maxillofac Surg. 1998; 56:1312–1326.
Article16. Choi JM, Kim HE, Lee IS. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials. 2000; 21:469–473.
Article17. Liu JQ, Luo ZS, Cui FZ, Duan XF, Peng LM. High-resolution transmission electron microscopy investigations of a highly adhesive hydroxyapatite coating/titanium interface fabricated by ion-beam-assisted deposition. J Biomed Mater Res. 2000; 52:115–118.
Article18. Le IS, Kim DH, Kim HE, Jung YC, Han CH. Biological performance of calcium phosphate films formed on commercially pure Ti by electron-beam evaporation. Biomaterials. 2002; 23:609–615.
Article19. Kim MK, Choi JY, Chae GJ, Jung UW, Kim ST, Lee IS, et al. The histometric analysis of osseointegration in hydroxyapatite surface dental implants by ion beam-assisted deposition. J Korean Acad Periodontol. 2008; 38:Suppl. 363–372.
Article20. Yoon HJ, Song JE, Um YJ, Chae GJ, Chung SM, Lee IS, et al. Effects of calcium phosphate coating to SLA surface implants by the ion-beam-assisted deposition method on self-contained coronal defect healing in dogs. Biomed Mater. 2009; 4:044107.
Article21. Chae GJ, Jung UW, Jung SM, Lee IS, Cho KS, Kim CK, et al. Healing of surgically created circumferential gap around Nano-coating surface dental implants in dogs. Surf Interface Anal. 2008; 40:184–187.
Article22. Um YJ, Song JE, Chae GJ, Jung UW, Chung SM, Lee IS, et al. The effect of post heat treatment of hydroxyapatite-coated implants on the healing of circumferential coronal defects in dogs. Thin Solid Film. 2009; 517:5375–5379.
Article23. Meredith N. Assessment of implant stability as a prognostic determinant. Int J Prosthodont. 1998; 11:491–501.24. Jung UW, Kim S, Kim YH, Cha JK, Lee IS, Choi SH. Osseointegration of dental implants installed without mechanical engagement: a histometric analysis in dogs. Clin Oral Implants Res. 2012; 23:1297–1301.
Article25. Blanco J, Alvarez E, Munoz F, Linares A, Cantalapiedra A. Influence on early osseointegration of dental implants installed with two different drilling protocols: a histomorphometric study in rabbit. Clin Oral Implants Res. 2011; 22:92–99.
Article26. Kim H, Choi SH, Chung SM, Li LH, Lee IS. Enhanced bone forming ability of SLA-treated Ti coated with a calcium phosphate thin film formed by e-beam evaporation. Biomed Mater. 2010; 5:044106.
Article27. Lee IS, Zhao BH, Lee GH, Choi SH, Chung SM. Industrial application of ion beam assisted deposition on medical implants. Surf Coat Technol. 2007; 201:5132–5137.
Article28. Lee IS, Kim HE, Kim SY. Studies on calcium phosphate coatings. Surf Coat Technol. 2000; 131:181–186.
Article29. Lang NP, Jepsen S. Working Group 4. Implant surfaces and design (Working Group 4). Clin Oral Implants Res. 2009; 20:Suppl 4. 228–231.
Article30. Lang NP, Berglundh T. Working Group 4 of Seventh European Workshop on Periodontology. Periimplant diseases: where are we now? Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011; 38:Suppl 11. 178–181.
Article31. Choi JY, Jung UW, Kim CS, Jung SM, Lee IS, Choi SH. Influence of nanocoated calcium phosphate on two different types of implant surfaces in different bone environment: an animal study. Clin Oral Implants Res. 2013; 24:1018–1022.
Article32. Cui FZ, Luo ZS, Feng QL. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J Mater Sci Mater Med. 1997; 8:403–405.33. Zablotsky M, Meffert R, Mills O, Burgess A, Lancaster D. The macroscopic, microscopic and spectrometric effects of various chemotherapeutic agents on the plasma-sprayed hydroxyapatite-coated implant surface. Clin Oral Implants Res. 1992; 3:189–198.
Article34. Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998; 11:391–401.35. Abrahamsson I, Berglundh T, Linder E, Lang NP, Lindhe J. Early bone formation adjacent to rough and turned endosseous implant surfaces: an experimental study in the dog. Clin Oral Implants Res. 2004; 15:381–392.
Article36. Novaes AB Jr, de Souza SL, de Barros RR, Pereira KK, Iezzi G, Piattelli A. Influence of implant surfaces on osseointegration. Braz Dent J. 2010; 21:471–481.
Article37. Feng B, Chen J, Zhang X. Interaction of calcium and phosphate in apatite coating on titanium with serum albumin. Biomaterials. 2002; 23:2499–2507.
Article38. Sul YT, Johansson CB, Albrektsson T. Oxidized titanium screws coated with calcium ions and their performance in rabbit bone. Int J Oral Maxillofac Implants. 2002; 17:625–634.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histomorphometric study of machined titanium implants and calcium phosphate coated titanium implants
- The Biological Effects of Calcium Phosphate Coated Implant for Osseointegration in Beagle Dogs
- Bone apposition on implants coated with calcium phosphate by ion beam assisted deposition in oversized drilled sockets: a histologic and histometric analysis in dogs
- The effect of fibronectin-coated implant on canine osseointegration
- The Effects of Citric Acid on HA coated Implant Surface