J Periodontal Implant Sci.
2011 Oct;41(5):242-247. 10.5051/jpis.2011.41.5.242.
The effect of fibronectin-coated implant on canine osseointegration
- Affiliations
-
- 1Department of Prosthodontics, Yonsei University College of Dentistry, Seoul, Korea.
- 2Department of Periodontology, Research Institute for Periodontal Regeneration, Yonsei University College of Dentistry, Seoul, Korea. shchoi726@yuhs.ac
- 3Department of Dental Biomaterials Science and Dental Research Institute, Seoul National University School of Dentistry, Seoul, Korea.
- 4Institute of Physics and Applied Physics, Atomic-Scale Surface Research Center, Yonsei University, Seoul, Korea.
- KMID: 1783619
- DOI: http://doi.org/10.5051/jpis.2011.41.5.242
Abstract
- PURPOSE
The purpose of this study was to characterize the osseointegration of the fibronectin-coated implant surface.
METHODS
Sand-blasted, large-grit, acid-etched (SLA) surface implants, with or without a thin calcium phosphate and fibronectin coating, were placed in edentulous mandibles of dogs 8 weeks after extraction. All dogs were sacrificed forhistological and histomorphometric evaluation after 4- and 8-week healing periods.
RESULTS
All types of implants were clinically stable without any mobility. Although the bone-to-implant contact and bone density of the SLA implants coated with calcium phosphate (CaP)/fibronectin were lower than the uncoated SLA implants, there were no significant differences between the uncoated SLA surface group and the SLA surface coated with CaP/fibronectin group.
CONCLUSIONS
Within the limits of this study, SLA surfaces coated with CaP/fibronectin were shown to have comparable bone-to-implant contact and bone density to uncoated SLA surfaces.
Keyword
MeSH Terms
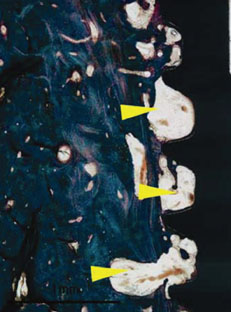
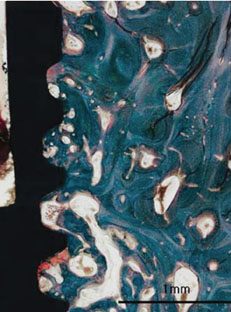
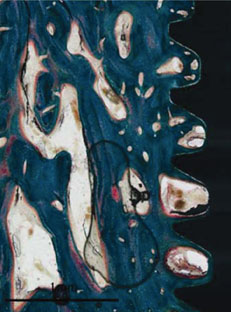
Figure
Cited by 1 articles
-
Early bone healing onto implant surface treated by fibronectin/oxysterol for cell adhesion/osteogenic differentiation: in vivo experimental study in dogs
Jung-Seok Lee, Jin-Hyuk Yang, Ji-Youn Hong, Ui-Won Jung, Hyeong-Cheol Yang, In-Seop Lee, Seong-Ho Choi
J Periodontal Implant Sci. 2014;44(5):242-250. doi: 10.5051/jpis.2014.44.5.242.
Reference
-
1. Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010. 28:198–206.
Article2. Atieh MA, Payne AG, Duncan WJ, de Silva RK, Cullinan MP. Immediate placement or immediate restoration/loading of single implants for molar tooth replacement: a systematic review and meta-analysis. Int J Oral Maxillofac Implants. 2010. 25:401–415.3. Bornstein MM, Hart CN, Halbritter SA, Morton D, Buser D. Early loading of nonsubmerged titanium implants with a chemically modified sand-blasted and acid-etched surface: 6-month results of a prospective case series study in the posterior mandible focusing on peri-implant crestal bone changes and implant stability quotient (ISQ) values. Clin Implant Dent Relat Res. 2009. 11:338–347.
Article4. Morton D, Bornstein MM, Wittneben JG, Martin WC, Ruskin JD, Hart CN, et al. Early loading after 21 days of healing of nonsubmerged titanium implants with a chemically modified sandblasted and acid-etched surface: two-year results of a prospective two-center study. Clin Implant Dent Relat Res. 2010. 12:9–17.
Article5. Oates TW, Valderrama P, Bischof M, Nedir R, Jones A, Simpson J, et al. Enhanced implant stability with a chemically modified SLA surface: a randomized pilot study. Int J Oral Maxillofac Implants. 2007. 22:755–760.6. Yoon HJ, Song JE, Um YJ, Chae GJ, Chung SM, Lee IS, et al. Effects of calcium phosphate coating to SLA surface implants by the ion-beam-assisted deposition method on self-contained coronal defect healing in dogs. Biomed Mater. 2009. 4:044107.
Article7. Kokubo T, Kim HM, Kawashita M, Nakamura T. Bioactive metals: preparation and properties. J Mater Sci Mater Med. 2004. 15:99–107.8. de Groot K, Wolke JG, Jansen JA. Calcium phosphate coatings for medical implants. Proc Inst Mech Eng H. 1998. 212:137–147.
Article9. Daculsi G, Laboux O, Malard O, Weiss P. Current state of the art of biphasic calcium phosphate bioceramics. J Mater Sci Mater Med. 2003. 14:195–200.10. Choi JM, Kim HE, Lee IS. Ion-beam-assisted deposition (IBAD) of hydroxyapatite coating layer on Ti-based metal substrate. Biomaterials. 2000. 21:469–473.
Article11. Lee IS, Whang CN, Kim HE, Park JC, Song JH, Kim SR. Various Ca/P ratios of thin calcium phosphate films. Mater Sci Eng C Biomim Mater Sens Syst. 2002. 22:15–20.
Article12. Hynes RO. Fibronectins. Sci Am. 1986. 254:42–51.
Article13. Horbett TA. Chapter 13 Principles underlying the role of adsorbed plasma proteins in blood interactions with foreign materials. Cardiovasc Pathol. 1993. 2:3 Suppl. 137–148.
Article14. Puleo DA, Nanci A. Understanding and controlling the bone-implant interface. Biomaterials. 1999. 20:2311–2321.
Article15. Cannas M, Denicolai F, Webb LX, Gristina AG. Bioimplant surfaces: binding of fibronectin and fibroblast adhesion. J Orthop Res. 1988. 6:58–62.16. Dean JW 3rd, Culbertson KC, D'Angelo AM. Fibronectin and laminin enhance gingival cell attachment to dental implant surfaces in vitro. Int J Oral Maxillofac Implants. 1995. 10:721–728.17. El-Ghannam A, Starr L, Jones J. Laminin-5 coating enhances epithelial cell attachment, spreading, and hemidesmosome assembly on Ti-6A1-4V implant material in vitro. J Biomed Mater Res. 1998. 41:30–40.
Article18. Roehlecke C, Witt M, Kasper M, Schulze E, Wolf C, Hofer A, et al. Synergistic effect of titanium alloy and collagen type I on cell adhesion, proliferation and differentiation of osteoblast-like cells. Cells Tissues Organs. 2001. 168:178–187.
Article19. Sugino A, Miyazaki T, Kawachi G, Kikuta K, Ohtsuki C. Relationship between apatite-forming ability and mechanical properties of bioactive PMMA-based bone cement modified with calcium salts and alkoxysilane. J Mater Sci Mater Med. 2008. 19:1399–1405.
Article20. Chen C, Lee IS, Zhang SM, Yang HC. Biomimetic apatite formation on calcium phosphate-coated titanium in Dulbecco's phosphate-buffered saline solution containing CaCl(2) with and without fibronectin. Acta Biomater. 2010. 6:2274–2281.
Article21. Hayakawa T, Yoshinari M, Nemoto K. Direct attachment of fibronectin to tresyl chloride-activated titanium. J Biomed Mater Res A. 2003. 67:684–688.
Article22. Park JM, Koak JY, Jang JH, Han CH, Kim SK, Heo SJ. Osseointegration of anodized titanium implants coated with fibroblast growth factor-fibronectin (FGF-FN) fusion protein. Int J Oral Maxillofac Implants. 2006. 21:859–866.23. do Serro AP, Fernandes AC, de Jesus Vieira Saramago B. Calcium phosphate deposition on titanium surfaces in the presence of fibronectin. J Biomed Mater Res. 2000. 49:345–352.
Article24. Weiss RE, Reddi AH. Synthesis and localization of fibronectin during collagenous matrix-mesenchymal cell interaction and differentiation of cartilage and bone in vivo. Proc Natl Acad Sci U S A. 1980. 77:2074–2078.
Article25. Saba TM, Jaffe E. Plasma fibronectin (opsonic glycoprotein): its synthesis by vascular endothelial cells and role in cardiopulmonary integrity after trauma as related to reticuloendothelial function. Am J Med. 1980. 68:577–594.
Article26. Tamada Y, Ikada Y. Effect of preadsorbed proteins on cell adhesion to polymer surfaces. J Colloid Interface Sci. 1993. 155:334–339.
Article27. Jönsson U, Ivarsson B, Lundström I, Berghem L. Adsorption behavior of fibronectin on well-characterized silica surfaces. J Colloid Interface Sci. 1982. 90:148–163.
Article28. Malmstrom HS, Fritz ME, Timmis DP, Van Dyke TE. Osseo-integrated implant treatment of a patient with rapidly progressive periodontitis. A case report. J Periodontol. 1990. 61:300–304.
Article29. Mombelli A, Lang NP. The diagnosis and treatment of peri-implantitis. Periodontol 2000. 1998. 17:63–76.
Article30. Davies JE. Mechanisms of endosseous integration. Int J Prosthodont. 1998. 11:391–401.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Teh Effect of Hydroxyapatite Coating on the Mechanical Strengths and Histologic Profiles of Porous Titanium Implants in Dogs
- A comparative experimental study of bone ingrowth and osseointegration in hydroxyapatite-coated vs. porous-coated implants
- The effect of purified human BMP with DLB(hBMP-I) on osseointegration of immediate titanium implants: cases report
- Comparative Study on Osseointegration of Calcium Metaphosphate (CMP) Coated Implant to RBM Implant in the Femur of Rabbits
- Bone apposition on implants coated with calcium phosphate by ion beam assisted deposition in oversized drilled sockets: a histologic and histometric analysis in dogs