J Korean Soc Transplant.
2012 Jun;26(2):120-124.
Use of Continuous Venovenous Hemodiafiltration to Enhance the Elimination of Serum Pentobarbital before Diagnosis of Brain Death
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Ajou University School of Medicine, Suwon, Korea. sicuab@hotmail.com
- 2Department of Pharmaceutical Service, Ajou University Hospital, Suwon, Korea.
- 3Department of Neurology, Ajou University School of Medicine, Suwon, Korea.
- 4Department of Neurosurgery, Ajou University School of Medicine, Suwon, Korea.
Abstract
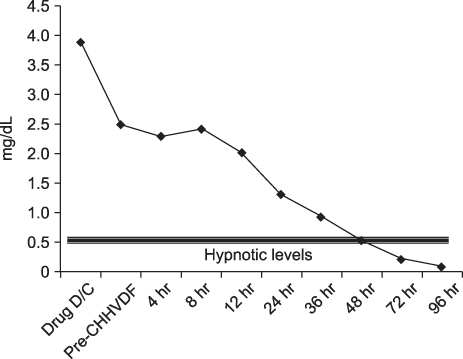
- Continuous venovenous hemodiafiltration (CVVHDF) was used to eliminate pentobarbital from the blood of a 30-year-old potentially brain dead male patient with traumatic intracranial hemorrhage after a motorcycle accident. The Acute Physiology and Chronic Health Evaluation (APACHE) II score of hospital day 1 was 24, but by day 8 it was 36, when the patient was considered to be brain dead. To control seizures and reduce intracranial pressure, pentobarbital had been administered in a continuous flow (2,880 mg/day for 5 days). Coma can be induced by pentobarbital at a serum level of 1~5 mg/dL. However, drug intoxication should be excluded from a brain death evaluation; therefore, the patient was not given any drug for approximately 88 hrs after ceasing pentobarbital in order for serum level to dip below 0.5 mg/dL (which is the hypnotic level). At 48 hours from CVVHDF, the pentobarbital level was close to the hypnotic level (0.1~0.5 mg/dL). Before stopping, the serum level of pentobarbital was 3.89 mg/dL and between 48 and 72 hours from CVVHDF, 4 cycles of pentobarbital half-life elimination (0.24 mg/dL) could be measured. Therefore, we suggest that in case of potential brain dead patients who have been administered pentobarbital, CVVHDF can enhance the elimination of pentobarbital from the circulatory system and shorten the waiting time for a brain death evaluation.
Keyword
MeSH Terms
Figure
Reference
-
1. Lacy CF, et al. American Pharmacists Association. Drug information handbook: a comprehensive resource for all clinicians and healthcare professionals, 19th ed. (2010-2011). c2010. Hudson, Ohio; [Washington, D.C.]: Lexi-Comp; American Pahrmacists Association;1209–1211.2. Roberts I. Barbiturates for acute traumatic brain injury. Cochrane Database Syst Rev. 2000. CD000033.
Article3. Chen HI, Malhotra NR, Oddo M, Oddo M, Heuer GG, Levine JM, et al. Barbiturate Infusion for intractable intracranial hypertension and its effect on brain oxygenation. Neurosurgery. 2008. 63:880–887. discussion 886-7.
Article4. Wijdicks EF. The diagnosis of brain death. N Engl J Med. 2001. 344:1215–1221.
Article5. Wikipedia. Pentobarbital [Internet]. 2010. cited 2010 Dec 20. San Francisco, CA: Wikimedia Foundation, Inc.;Available from: http://en.wikipedia.org/wiki/Pentobarbital.6. Berman LB, Vogelsang P. Removal rates for barbiturates using two types of peritoneal dialysis. N Engl J Med. 1964. 270:77–80.
Article7. Hudson JB, Dennis AJ Jr, Hobbs DR, Sussman HC. Extended hemodialysis in short acting barbiturate poisoning: case report. South Med J. 1969. 62:457–460.8. Chow-Tung E, Lau AH, Vidyasagar D, John EG. Clearance of phenobarbital by peritoneal dialysis in a neonate. Clin Pharm. 1982. 1:268–271.9. Wermeling D, Record K, Bell R, Porter W, Blouin R. Hemodialysis clearance of pentobarbital during continuous infusion. Ther Drug Monit. 1985. 7:485–487.10. Porto I, John EG, Heilliczer J. Removal of phenobarbital during continuous cycling peritoneal dialysis in a child. Pharmacotherapy. 1997. 17:832–835.11. Lal R, Faiz S, Garg RK, Baweja KS, Guntupalli J, Finkel KW. Use of continuous venovenous hemodiafiltration in a case of severe phenobarbital poisoning. Am J Kidney Dis. 2006. 48:e13–e15.
Article12. Harvey SC. Goodman LS, Gilman A, editors. Hypnotics and sedatives: the barbiturates. The pharmacological basis of therapeutics. 1975. 5th ed. New York: Macmillan;102–123.13. Trevor AJ, Way WL. Katzung BG, editor. Sedative-Hypnotics. Basic and clinical pharmacology. 1982. Los Altos: Lange;221–230.14. Grattan-Smith PJ, Butt W. Suppression of brainstem reflexes in barbiturate coma. Arch Dis Child. 1993. 69:151–152.
Article15. Yang KL, Dantzker DR. Reversible brain death. A manifestation of amitriptyline overdose. Chest. 1991. 99:1037–1038.16. Venkataraman R, Song M, Lynas R, Kellum JA. Hemoadsorption to improve organ recovery from brain-dead organ donors: a novel therapy for a novel indication? Blood purify. 2004. 22:143–149.
Article17. Dellinger RP, Levy MM, Carlet JM, Bion J, Parker MM, Jaeschke R, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008. 36:296–327. Erratum in: Crit Care Med 2008;36:1394-6.
Article18. Belloma R, D'Intini V, et al. Fink MP, Abraham E, editors. Renal replacement therapy in the ICU. Textbook of critical care. 2005. 5th ed. Philadelpia: Elsevier Saunders;1151–1158.19. Cole L, Bellomo R, Hart G, Journois D, Davenport P, Tipping P, et al. A phase II randomized, controlled trial of continuous hemofiltration in sepsis. Crit Care Med. 2002. 30:100–106.
Article20. Bironneau E, Garrec F, Kergueris MF, Testa A, Nicolas F. Hemodiafiltration in pentobarbital poisoning. Ren Fail. 1996. 18:299–303.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Continuous Venovenous Hemodiafiltration in the Treatment of Neonatal Hyperammonemia Due to Methylmalonic Acidemia
- Delayed Continuous Venovenous Hemodiafiltration in Chronic Lithium Intoxication
- Successful Treatment of Severe Lactic Acidosis by Continuous Venovenous Hemodiafiltration
- A Case of Acute on Chronic Salicylate Poisoned Elderly Patient with Early Utilization of Continuous Venovenous Hemodiafiltration: A Case Report
- A Case of Acute Lithium Intoxication Treated with Continuous Venovenous Hemodiafiltration