J Korean Soc Spine Surg.
2003 Dec;10(4):283-289.
Spinal Fusion by Percutaneous OP-1 Gene Delivery
- Affiliations
-
- 1Department of Orthopaedic Surgery, Kuri Hospital, College of Medicine, Hanyang University. hyparkys@hanyang.ac.kr
- 2Department of Biomedical Engineering, Johns Hopkins University.
- 3Department of Orthopaedic Surgery, Johns Hopkins University.
Abstract
- STUDY DESIGN: An in-vivo experiment.
OBJECTIVES
To evaluate the feasibility of achieving bone formation by percutaneous gene delivery, with plasmid DNA encoding BMP-7(OP-1). SUMMARY OF LITERATURE REVIEW: Currently, the preferred method for posterolateral spinal fusion involves decortication of the transverse process, followed by a graft of autogenous bone harvested from the iliac crest. Unfortunately, this procedure suffers from significant morbidity, including blood loss, infection and persistent pain at the harvest site. MATERIAL AND METHODS: 24 Sprague-Dawley rats, weighing approximately 250~300 g, were used. The percutaneous injection was attempted above both the L5 transverse processes. The animals were divided into three groups, according to the injection materials: 1) OP-1 gene/collagen, 2) recombinant OP-1 protein/collagen and 3) control of PBS/collagen. At 2 and 4 weeks post-injection, the animals were sacrificed. The gross, radiological and histological findings were analyzed.
RESULTS
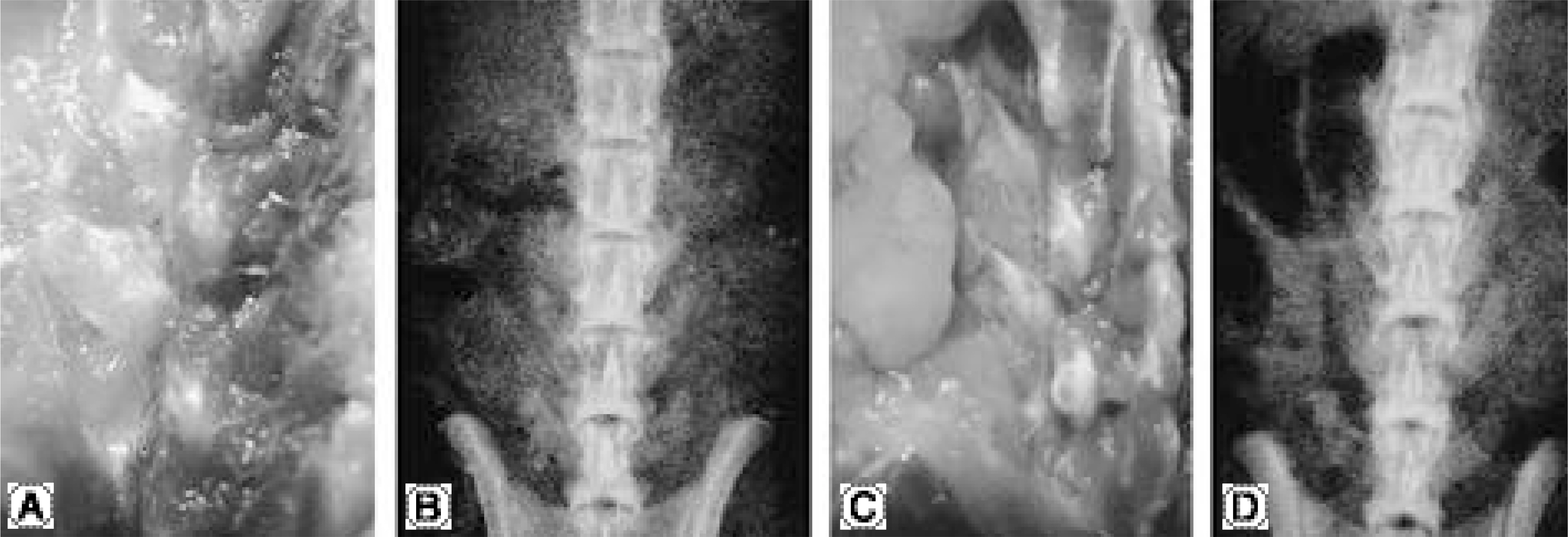
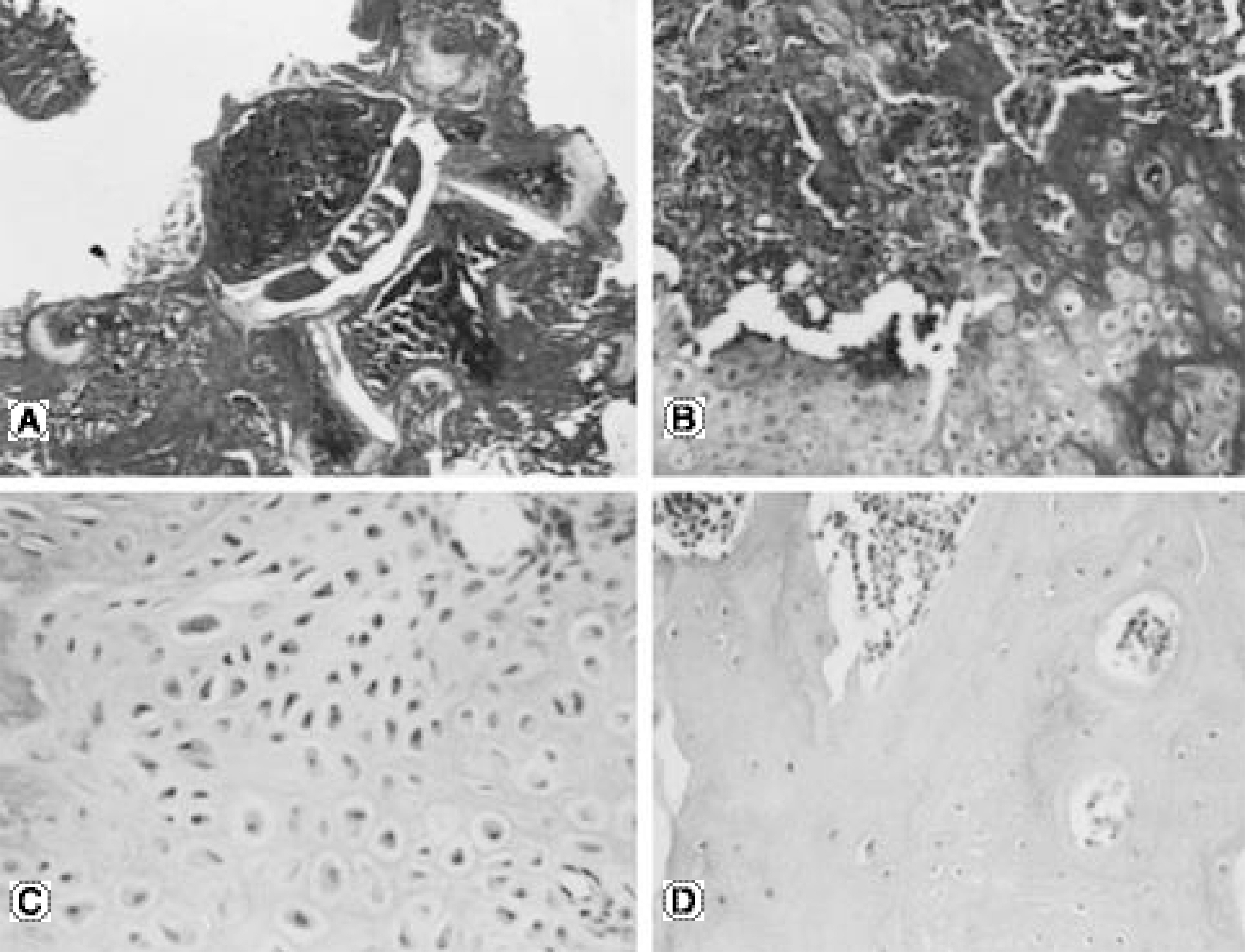
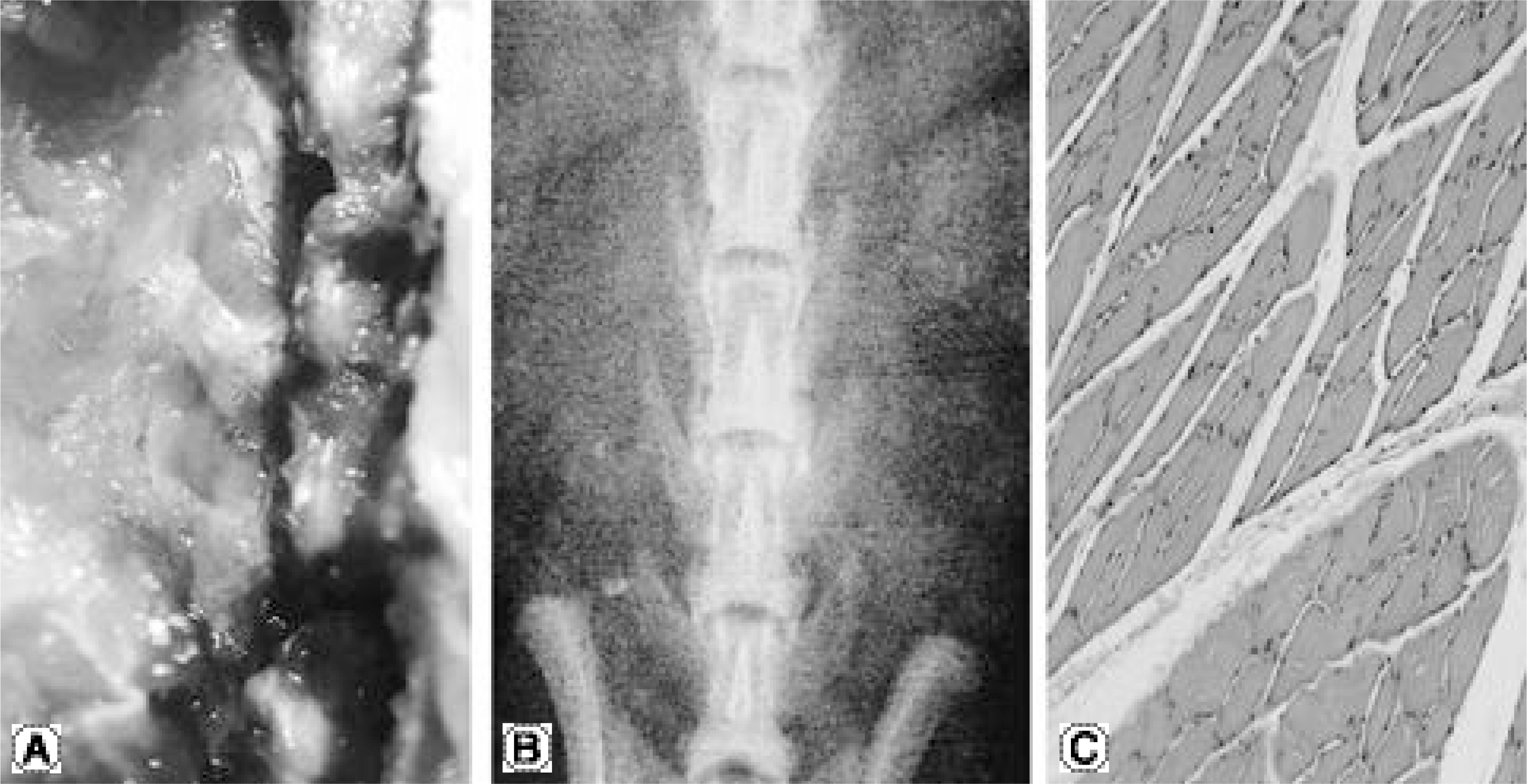
No bone was detected grossly by manual palpation or radiography in the groups receiving OP-1 gene/collagen at either time point. The histological findings revealed the initiation of endochondral bone formation within the paraspinal muscle, directly above the L5 transverse process. In the rhOP-1 protein/collagen groups, the gross, radiological and histological findings revealed extensive cartilage and bone formation at both 2 and 4 weeks.
CONCLUSION
In conclusion, the authors confirmed the feasibility of achieving bone formation by percutaneous gene delivery, with plasmid DNA encoding BMP-7(OP-1).
Keyword
MeSH Terms
Figure
Reference
-
1). Damien CJ, Parsons JR. Bone graft and bone graft substitutes. A review of current technology applications Journal of Applied Biomaterials. 1991; 2:187–208.2). Younger EM, Chapman MW. Morbidity at bone graft donor site. J Orthop Trauma. 1989; 3:192–195.3). Summers BN, Eisenstein SM. Donor site pain from the ilium. A complication of lumbar spine fusion. J Bone Joint Surg. 1989; 71B:677–680.
Article4). Steinmann JC, Herkowitz HN. Pseudarthrosis of the spine. Clin Orthop. 1992; 284:80–90.
Article5). Cook SD, Dalton JE, Tan EH, Whitecloud III TS, Rueger DC. In vivo evaluation of recombinant human osteogenic protein(rhOP-1) implants as a bone graft substitute for spinal fusions. Spine. 1994; 19:1655–1663.6). Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friendlaender GE. Evaluation of op-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001; 26:127–133.
Article7). Salamon ML, Althausen PL, Gupta MC, Laubach J. The effects of BMP-7 in a rat posterolateral intertransverse process fusion model. J Spinal Disord. 2003; 16:90–95.
Article8). Schimandle JH, Boden SD, Hutton WC. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine. 1995; 20:1326–1337.
Article9). Boden SD, Martin GJ, Horton WC, Truss TL, Sandhu HS. Laparoscopic anterior spinal arthrodesis with rh BMP-2 in a titanium interbody threaded cage. J Spinal Disord. 1998; 11:95–101.10). Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages: Definitive evidence of osteoinduction in humans. Spine. 2000; 25:376–381.11). Sandhu HS, Kanim LEA, Kabo JM et al.E f fe ct iv e doses of recombinant bone morphogenetic protein-2 in experimental spinal fusion. Spine. 1996; 21:2115–2122.12). Alden TD, Pittman DD, Beres EJ et al.Percutaneous spinal fusion using bone morphogenetic protein-2 gene therapy. J. Neurosurg. 1999; 90:109–114.13). Sandhu HS, Khan SN. Animal models for preclinical assessment of bone morphogenetic proteins in the spine. Spine. 2002; 27:S32–S38.
Article14). Boden SD. Biology of lumbar spine fusion and use of bone graft substitutes: present, future, and next generation. Tissue Engineering. 2000; 6:383–399.
Article15). Danko I, Williams P, Herweijer H et al.High expression of naked plasmid DNA in muscles of young rodents. Human Mo ecular Genetics. 1997; 6:1435–1443.16). Wolff JA, Dowty ME, Jiao S, et al. Expression of naked plasmids by cultured myotubes and entry of plasmids into T tubules and caveolae of mammalian skeletal muscle. Journal of Cell Science. 1992; 103:1249–1259.
Article17). Wolff JA. Direct gene transfer into mouse muscle in vivo. Science. 1990; 247:1465–1468.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Percutaneous Pedicle Screw Fixation in the Lumbar Spine
- Future Development of Interbody Fusion Cages
- Etiology, diagnosis, and treatment of iatrogenic spinal deformity
- Abducens Nerve Palsy after Lumbar Spinal Fusion Surgery with Inadvertent Dural Tearing
- The Use of the Longitudinal Traction and Anterior Spinal Fusion in A Patient with the Tuberculous Kyphosis