Korean J Urol.
2011 Feb;52(2):136-141.
Leptin Enhances Nitric Oxide-Dependent Relaxation of the Clitoral Corpus Cavernosum
- Affiliations
-
- 1Department of Urology, Seoul Medical Center, Seoul, Korea.
- 2Department of Urology and Advanced Urogenital Diseases Research Center, College of Medicine, Chung-Ang University, Seoul, Korea. uromyung@cau.ac.kr
- 3Department of Physiology, College of Medicine, Chung-Ang University, Seoul, Korea.
- 4Department of Urology, Catholic University Medical Center, Seoul, Korea.
Abstract
- PURPOSE
The effects of leptin on female sexual behaviors are controversial, and studies on this topic are limited. The objectives of this study were to evaluate the direct effects of leptin on clitoral vasoreactivity in vitro and to determine the mechanism of action.
MATERIALS AND METHODS
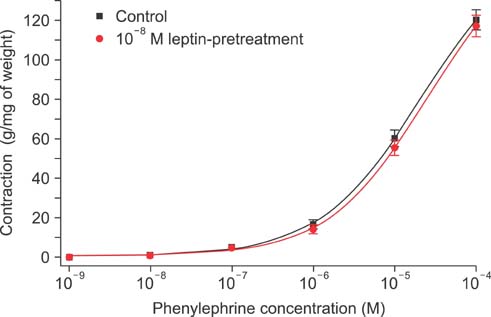
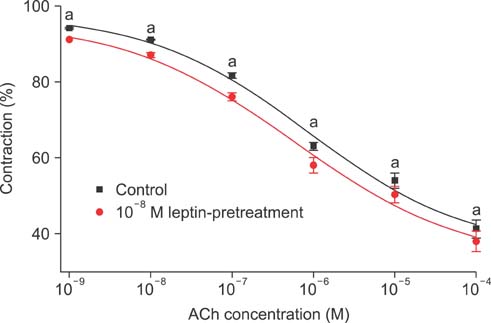
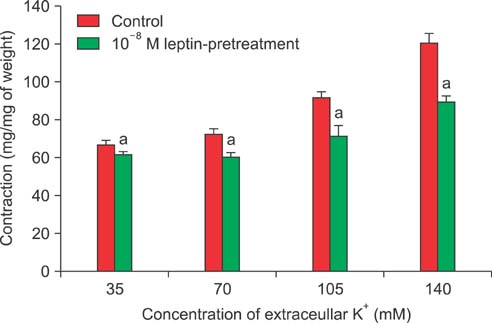
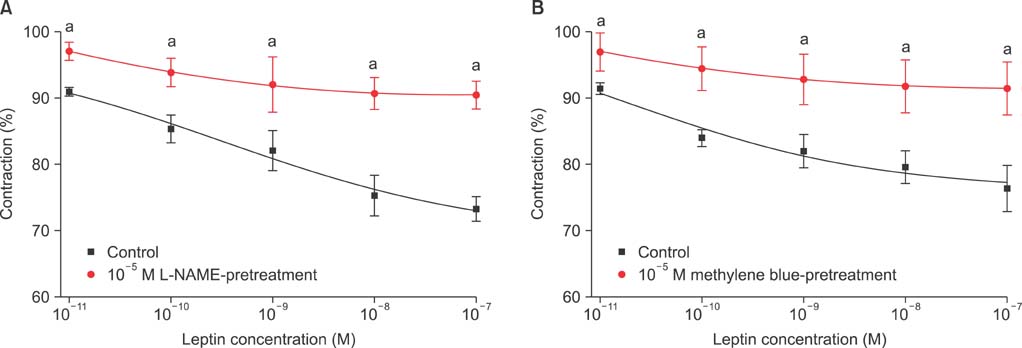
Isometric tension studies were conducted to determine the effects of pretreatment with leptin (10(-8) M) on the contractile responses of rabbit clitoral corpus cavernosal smooth muscle strips. The effects of leptin were assessed on precontraction induced by phenylephrine (PE; 10(-9)-10(-4) M) and KCl (35-140 mM). We also examined the effect of leptin on relaxation induced by acetylcholine (ACh; 10(-9)-10(-4) M), verapamil (10(-10)-10(-6) M), and sodium nitroprusside (10(-9)-10(-4) M) in PE-precontracted (10(-5) M) strips.
RESULTS
Leptin enhanced ACh-induced relaxation in PE-precontracted strips. L-NAME pretreatment significantly reduced the effect of leptin on ACh-induced relaxation, whereas L-arginine potentiated the effect of leptin. Leptin decreased the KCl-induced contractile responses. Leptin increased verapamil-induced relaxation responses. The relaxation effects of leptin on KCl-induced contraction were inhibited by 10(-5) M methylene blue and L-NAME pretreatment.
CONCLUSIONS
A high concentration of leptin enhances ACh-dependent relaxation in clitoral cavernosal smooth muscles. These relaxation effects of leptin may occur through an NO-dependent mechanism and voltage-dependent calcium channel blockade.
Keyword
MeSH Terms
-
Acetylcholine
Arginine
Calcium Channels
Clitoris
Contracts
Female
Humans
Leptin
Methylene Blue
Muscle, Smooth
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitroprusside
Phenylephrine
Relaxation
Sexual Behavior
Verapamil
Acetylcholine
Arginine
Calcium Channels
Leptin
Methylene Blue
NG-Nitroarginine Methyl Ester
Nitric Oxide
Nitroprusside
Phenylephrine
Verapamil
Figure
Reference
-
1. Rodríguez A, Fortuño A, Gómez-Ambrosi J, Zalba G, Díez J, Frühbeck G. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007. 148:324–331.2. Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996. 379:632–635.3. Kimura K, Tsuda K, Baba A, Kawabe T, Boh-oka S, Ibata M, et al. Involvement of nitric oxide in endothelium-dependent arterial relaxation by leptin. Biochem Biophys Res Commun. 2000. 273:745–749.4. Quehenberger P, Exner M, Sunder-Plassmann R, Ruzicka K, Bieglmayer C, Endler G, et al. Leptin induces endothelin-1 in endothelial cells in vitro. Circ Res. 2002. 90:711–718.5. Söderberg S, Ahrén B, Jansson JH, Johnson O, Hallmans G, Asplund K, et al. Leptin is associated with increased risk of myocardial infarction. J Intern Med. 1999. 246:409–418.6. Leyva F, Anker SD, Egerer K, Stevenson JC, Kox WJ, Coats AJ. Hyperleptinaemia in chronic heart failure. Relationships with insulin. Eur Heart J. 1998. 19:1547–1551.7. Schulze PC, Kratzsch J, Linke A, Schoene N, Adams V, Gielen S, et al. Elevated serum levels of leptin and soluble leptin receptor in patients with advanced chronic heart failure. Eur J Heart Fail. 2003. 5:33–40.8. Wallace AM, McMahon AD, Packard CJ, Kelly A, Shepherd J, Gaw A, et al. Plasma leptin and the risk of cardiovascular disease in the west of Scotland coronary prevention study (WOSCOPS). Circulation. 2001. 104:3052–3056.9. Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, et al. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin-resistant men. Hypertension. 1999. 34:1047–1052.10. Ehrlich S, Burghardt R, Schneider N, Hein J, Weiss D, Pfeiffer E, et al. Leptin and its associations with measures of psychopathology in patients with anorexia nervosa. J Neural Transm. 2009. 116:109–115.11. Fox AS, Olster DH. Effects of intracerebroventricular leptin administration on feeding and sexual behaviors in lean and obese female Zucker rats. Horm Behav. 2000. 37:377–387.12. Levin LJ. VIP, vagina, clitoral and periurethral glans--an update on human female genital arousal. Exp Clin Endocrinol. 1991. 98:61–69.13. Azadzoi KM, Siroky MB. Neurologic factors in female sexual function and dysfunction. Korean J Urol. 2010. 51:443–449.14. Patel SB, Reams GP, Spear RM, Freeman RH, Villarreal D. Leptin: linking obesity, the metabolic syndrome, and cardiovascular disease. Curr Hypertens Rep. 2008. 10:131–137.15. Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, et al. Biological action of leptin as an angiogenic factor. Science. 1998. 281:1683–1686.16. Bouloumié A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998. 83:1059–1066.17. Park HY, Kwon HM, Lim HJ, Hong BK, Lee JY, Park BE, et al. Potential role of leptin in angiogenesis: leptin induces endothelial proliferation and expression of matrix metalloproteinases in vivo and in vitro. Exp Mol Med. 2001. 33:95–102.18. Rahmouni K, Haynes WG. Endothelial effects of leptin: implications in health and diseases. Curr Diab Rep. 2005. 5:260–266.19. Ren J. Leptin and hyperleptinemia - from friend to foe for cardiovascular function. J Endocrinol. 2004. 181:1–10.20. Musicki B, Liu T, Lagoda GA, Bivalacqua TJ, Strong TD, Burnett AL. Endothelial nitric oxide synthase regulation in female genital tract structures. J Sex Med. 2009. 6:Suppl 3. 247–253.21. Uckert S, Mayer ME, Jonas U, Stief CG. Potential future options in the pharmacotherapy of female sexual dysfunction. World J Urol. 2006. 24:630–638.22. Gragasin FS, Michelakis ED, Hogan A, Moudgil R, Hashimoto K, Wu X, et al. The neurovascular mechanism of clitoral erection: nitric oxide and cGMP-stimulated activation of BKCa channels. FASEB J. 2004. 18:1382–1391.23. Sullivan M, Thompson CS, Mikhailidis DP, Morgan RJ, Angelini GD, Jeremy JY. Differential alterations of prostacyclin, cyclic AMP and cyclic GMP formation in the corpus cavernosum of the diabetic rabbit. Br J Urol. 1998. 82:578–584.24. Takizawa H, Ura N, Saitoh S, Wang L, Higashiura K, Takagi S, et al. Gender difference in the relationships among hyperleptinemia, hyperinsulinemia, and hypertension. Clin Exp Hypertens. 2001. 23:357–368.25. Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996. 81:3424–3427.26. Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996. 334:292–295.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Nitric Oxide in the Relaxation of Canine Corpus Cavernosum Smooth Muscle
- Effects of Electrical Field Stimulation of the Isolated Corpus Cavernosum from Hypertensive Rats
- Effect of Korean Red Ginseng on the Relaxation of Clitoral Corpus Cavernosum in Rabbit
- Mechanism of Action of Endothelium-Dependent Relaxation Substances on Rabbit Corpus Cavernosum
- The Effect of Nitric Oxide on Cat Corpus Cavernosum Relaxation Under Hypoxia (In vivo Study)