Clin Exp Otorhinolaryngol.
2009 Jun;2(2):55-60. 10.3342/ceo.2009.2.2.55.
Animal Models of Cancer in the Head and Neck Region
- Affiliations
-
- 1Department of Otolaryngology, School of Medicine, University of Pittsburgh, USA. kimsw2@upmc.edu
- KMID: 2007185
- DOI: http://doi.org/10.3342/ceo.2009.2.2.55
Abstract
- Animal models that resemble the cancers of the head and neck region are of paramount importance in studying the carcinogenesis of these diseases. Although several methods for modeling cancer in the head and neck are available, none are fully satisfactory. Subcutaneous xenograft models of cancer in nude mice are often used in preclinical studies. However, these models are problematic in several aspects as they lack the specific interactions that exist between the tumor cells and their native environment. Establishment of tumors at the orthotopic sites restore these distinct patterns of interactions between the tumor and the host organs that are lost or altered when the tumors are established in ectopic sites. With regard to the transgenic model of cancer in the head and neck region, it should be kept in mind that the transgene used to drive the malignant transformation may not be representative of the carcinogenic process found in human tumors. Low penetrance of tumor formation also translates into high cost and time commitment in performing studies with transgenic models. In this review, we will discuss some of the commonly used methods for modeling cancer in the head and neck region including squamous cell carcinoma of the head and neck as well as thyroid carcinoma.
MeSH Terms
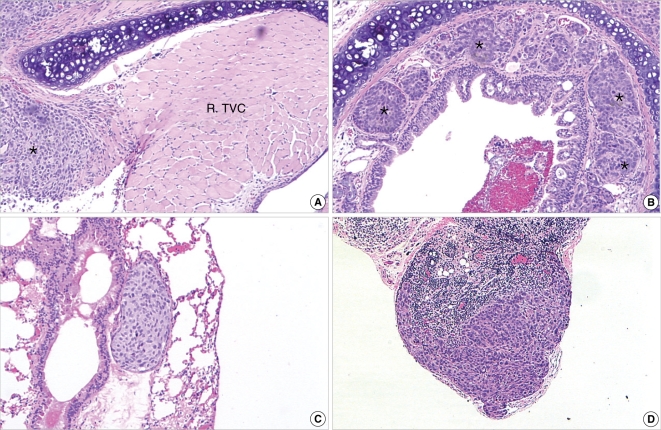
Figure
Cited by 1 articles
-
The Promoting Effect of Carbamide Peroxide Teeth Bleaching Gel in a Preclinical Model of Head and Neck Cancer in Hamster Buccal Pouch
Vinícius Faccin Bampi, Wadson Ferreira Vilela, Reggiani Vilela Gonçalves, Maria Gabriela Tavares Rheingantz, Luiz Fernando Minello, Jefferson Luis Braga da Silva, Laura Beatriz Oliveira de Oliveira
Clin Exp Otorhinolaryngol. 2014;7(3):210-215. doi: 10.3342/ceo.2014.7.3.210.
Reference
-
1. Fidler IJ. Rationale and methods for the use of nude mice to study the biology and therapy of human cancer metastasis. Cancer Metastasis Rev. 1986; 5(1):29–49. PMID: 2942306.
Article2. Killion JJ, Radinsky R, Fidler IJ. Orthotopic models are necessary to predict therapy of transplantable tumors in mice. Cancer Metastasis Rev. 1998-1999; 17(3):279–284. PMID: 10352881.3. Kubota T. Metastatic models of human cancer xenografted in the nude mouse: the importance of orthotopic transplantation. J Cell Biochem. 1994; 9. 56(1):4–8. PMID: 7806591.
Article4. Singh RK, Bucana CD, Gutman M, Fan D, Wilson MR, Fidler IJ. Organ site-dependent expression of basic fibroblast growth factor in human renal cell carcinoma cells. Am J Pathol. 1994; 8. 145(2):365–374. PMID: 8053494.5. Fidler IJ, Wilmanns C, Staroselsky A, Radinsky R, Dong Z, Fan D. Modulation of tumor cell response to chemotherapy by the organ environment. Cancer Metastasis Rev. 1994; 6. 13(2):209–222. PMID: 7923551.
Article6. Dong Z, Radinsky R, Fan D, Tsan R, Bucana CD, Wilmanns C, et al. Organ-specific modulation of steady-state mdr gene expression and drug resistance in murine colon cancer cells. J Natl Cancer Inst. 1994; 6. 86(12):913–920. PMID: 7910854.
Article7. Radinsky R, Beltran PJ, Tsan R, Zhang R, Cone RD, Fidler IJ. Transcriptional induction of the melanocyte-stimulating hormone receptor in brain metastases of murine K-1735 melanoma. Cancer Res. 1995; 1. 55(1):141–148. PMID: 7805024.8. Salley JJ. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954; 4. 33(2):253–262. PMID: 13152263.
Article9. Lin LM, Chen YK, Lai DL, Huang YL. Minimal arecaidine concentrations showing a promotion effect during DMBA-induced hamster cheek pouch carcinogenesis. J Oral Pathol Med. 1996; 2. 25(2):65–68. PMID: 8667258.
Article10. Kim TW, Chen Q, Shen X, Regezi JA, Ramos DM, Tanaka H, et al. Oral mucosal carcinogenesis in SENCAR mice. Anticancer Res. 2002; Sep–Oct. 22(5):2733–2740. PMID: 12529989.11. Wong DT, Gallagher GT, Gertz R, Chang AL, Shklar G. Transforming growth factor alpha in chemically transformed hamster oral keratinocytes. Cancer Res. 1988; 6. 48(11):3130–3134. PMID: 2452686.12. Schwartz JL, Shklar G. Glutathione inhibits experimental oral carcinogenesis, p53 expression, and angiogenesis. Nutr Cancer. 1996; 26(2):229–236. PMID: 8875560.
Article13. Gimenez-Conti IB, Bianchi AB, Stockman SL, Conti CJ, Slaga TJ. Activating mutation of the Ha-ras gene in chemically induced tumors of the hamster cheek pouch. Mol Carcinog. 1992; 5(4):259–263. PMID: 1497802.
Article14. Robles AI, Gimenez-Conti IB, Roop D, Slaga TJ, Conti CJ. Low frequency of codon 61 Ha-ras mutations and lack of keratin 13 expression in 7,12-dimethylbenz[a]-anthracene-induced hamster skin tumors. Mol Carcinog. 1993; 7(2):94–98. PMID: 7681292.
Article15. Schwartz JL, Antoniades DZ, Zhao S. Molecular and biochemical reprogramming of oncogenesis through the activity of prooxidants and antioxidants. Ann N Y Acad Sci. 1993; 5. 686:262–278. PMID: 8512252.
Article16. Wallenius K, Lekholm U. Oral cancer in rats induced by the water-soluble carcinogen 4-nitrochinoline N-oxide. Odontol Revy. 1973; 24(1):39–48. PMID: 4514062.17. Yuan B, Heniford BW, Ackermann DM, Hawkins BL, Hendler FJ. Harvey ras (H-ras) point mutations are induced by 4-nitroquinoline-1-oxide in murine oral squamous epithelia, while squamous cell carcinomas and loss of heterozygosity occur without additional exposure. Cancer Res. 1994; 10. 54(20):5310–5317. PMID: 7923158.18. Osugi Y. p53 expression in various stages of 4-nitroquinoline 1-oxide induced carcinoma in the rat tongue. J Osaka Dent Univ. 1996; 12. 30(1-2):29–35. PMID: 9485768.19. Sakaki T, Tamura I, Kadota H, Kakudo K. Changing expression of E- and P-cadherin during rat tongue carcinogenesis induced by 4-nitroquinoline 1-oxide. J Oral Pathol Med. 2003; 10. 32(9):530–537. PMID: 12969227.
Article20. Nishimura A. Changes in Bcl-2 and Bax expression in rat tongue during 4-nitroquinoline 1-oxide-induced carcinogenesis. J Dent Res. 1999; 6. 78(6):1264–1269. PMID: 10371251.
Article21. Nauta JM, Roodenburg JL, Nikkels PG, Witjes MJ, Vermey A. Comparison of epithelial dysplasia: the 4NQO rat palate model and human oral mucosa. Int J Oral Maxillofac Surg. 1995; 2. 24(1 Pt 1):53–58. PMID: 7782642.22. Pathak I, Davis NL, Hsiang YN, Quenville NF, Palcic B. Detection of squamous neoplasia by fluorescence imaging comparing porfimer sodium fluorescence to tissue autofluorescence in the hamster cheek-pouch model. Am J Surg. 1995; 11. 170(5):423–426. PMID: 7485724.
Article23. Wang CY, Tsai T, Chen HC, Chang SC, Chen CT, Chiang CP. Autofluorescence spectroscopy for in vivo diagnosis of DMBA-induced hamster buccal pouch pre-cancers and cancers. J Oral Pathol Med. 2003; 1. 32(1):18–24. PMID: 12558954.24. Fitch KA, Somers KD, Schechter GL. Wolf GT, Carey TE, editors. The development of a head and neck tumor model in nude mouse. Head and Neck Oncology Research. 1988. Amsterdam: Kugler Publication;p. 187–190.25. Dinesman A, Haughey B, Gates GA, Aufdemorte T, Von Hoff DD. Development of a new in vivo model for head and neck cancer. Otolaryngol Head Neck Surg. 1990; 11. 103(5 Pt 1):766–774. PMID: 2126099.
Article26. Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002; 1. 8(1):293–298. PMID: 11801572.27. O'Malley BW Jr, Cope KA, Johnson CS, Schwartz MR. A new immunocompetent murine model for oral cancer. Arch Otolaryngol Head Neck Surg. 1997; 1. 123(1):20–24. PMID: 9006499.28. Cui N, Nomura T, Noma H, Yokoo K, Takagi R, Hashimoto S, et al. Effect of YM529 on a model of mandibular invasion by oral squamous cell carcinoma in mice. Clin Cancer Res. 2005; 4. 11(7):2713–2719. PMID: 15814653.
Article29. Caulin C, Nguyen T, Longley MA, Zhou Z, Wang XJ, Roop DR. Inducible activation of oncogenic K-ras results in tumor formation in the oral cavity. Cancer Res. 2004; 8. 64(15):5054–5058. PMID: 15289303.30. Vitale-Cross L, Amornphimoltham P, Fisher G, Molinolo AA, Gutkind JS. Conditional expression of K-ras in an epithelial compartment that includes the stem cells is sufficient to promote squamous cell carcinogenesis. Cancer Res. 2004; 12. 64(24):8804–8807. PMID: 15604235.
Article31. Raimondi AR, Molinolo A, Gutkind JS. Rapamycin prevents early onset of tumorigenesis in an oral-specific K-ras and p53 two-hit carcinogenesis model. Cancer Res. 2009; 5. 69(10):4159–4166. PMID: 19435901.
Article32. Moral M, Segrelles C, Lara MF, Martinez-Cruz AB, Lorz C, Santos M, et al. Akt activation synergizes with Trp53 loss in oral epithelium to produce a novel mouse model for head and neck squamous cell carcinoma. Cancer Res. 2009; 2. 69(3):1099–1108. PMID: 19176372.33. Saranath D, Chang SE, Bhoite LT, Panchal RG, Kerr IB, Mehta AR, et al. High frequency mutation in codons 12 and 61 of H-ras oncogene in chewing tobacco-related human oral carcinoma in India. Br J Cancer. 1991; 4. 63(4):573–578. PMID: 2021541.
Article34. Munirajan AK, Mohanprasad BK, Shanmugam G, Tsuchida N. Detection of a rare point mutation at codon 59 and relatively high incidence of H-ras mutation in Indian oral cancer. Int J Oncol. 1998; 11. 13(5):971–974. PMID: 9772288.
Article35. Kim S, Park YW, Schiff BA, Doan DD, Yazici Y, Jasser SA, et al. An orthotopic model of anaplastic thyroid carcinoma in athymic nude mice. Clin Cancer Res. 2005; 3. 11(5):1713–1721. PMID: 15755992.
Article36. Ahn SH, Henderson Y, Kang Y, Chattopadhyay C, Holton P, Wang M, et al. An orthotopic model of papillary thyroid carcinoma in athymic nude mice. Arch Otolaryngol Head Neck Surg. 2008; 2. 134(2):190–197. PMID: 18283163.
Article37. Cohen Y, Xing M, Mambo E, Guo Z, Wu G, Trink B, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst. 2003; 4. 16. 95(8):625–627. PMID: 12697856.
Article38. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002; 6. 417(6892):949–954. PMID: 12068308.
Article39. Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab. 2005; 12. 90(12):6373–6379. PMID: 16174717.
Article40. Knauf JA, Ma X, Smith EP, Zhang L, Mitsutake N, Liao XH, et al. Targeted expression of BRAFV600E in thyroid cells of transgenic mice results in papillary thyroid cancers that undergo dedifferentiation. Cancer Res. 2005; 5. 65(10):4238–4245. PMID: 15899815.
Article41. Tallini G, Asa SL. RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol. 2001; 11. 8(6):345–354. PMID: 11707626.
Article42. Lam AK, Montone KT, Nolan KA, Livolsi VA. Ret oncogene activation in papillary thyroid carcinoma: prevalence and implication on the histological parameters. Hum Pathol. 1998; 6. 29(6):565–568. PMID: 9635675.
Article43. Nakazawa T, Kondo T, Kobayashi Y, Takamura N, Murata S, Kameyama K, et al. RET gene rearrangements (RET/PTC1 and RET/PTC3) in papillary thyroid carcinomas from an iodine-rich country (Japan). Cancer. 2005; 9. 104(5):943–951. PMID: 16015630.44. Kondo T, Ezzat S, Asa SL. Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nat Rev Cancer. 2006; 4. 6(4):292–306. PMID: 16557281.
Article45. Jhiang SM, Sagartz JE, Tong Q, Parker-Thornburg J, Capen CC, Cho JY, et al. Targeted expression of the ret/PTC1 oncogene induces papillary thyroid carcinomas. Endocrinology. 1996; 1. 137(1):375–378. PMID: 8536638.
Article46. La Perle KM, Jhiang SM, Capen CC. Loss of p53 promotes anaplasia and local invasion in ret/PTC1-induced thyroid carcinomas. Am J Pathol. 2000; 8. 157(2):671–677. PMID: 10934169.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A clinical study on neck dissection in cases of head and neck cancer
- Current Status of Immunotherapy in Head and Neck Cancer
- Personalized Treatment of Head and Neck Cancers and the Role of Head and Neck Surgeons
- A study for the incidence of neck metastasis in head and neck cancer
- Experimental Animal Models of Pancreatic Diseases