Nutr Res Pract.
2016 Feb;10(1):26-32. 10.4162/nrp.2016.10.1.26.
Vitamin A supplementation modifies the antioxidant system in rats
- Affiliations
-
- 1Department of Food and Nutrition, Yeungnam University, 280 Daehak-ro, Gyeongsan, Gyeongbuk 38541, Korea. jsseo@ynu.ac.kr
- KMID: 2313897
- DOI: http://doi.org/10.4162/nrp.2016.10.1.26
Abstract
- BACKGROUND/OBJECTIVES
It has been shown that vitamin A supplementation has different effects on skeletal health and the antioxidant system. Deficiency or excess of this vitamin can lead to health problems. Vitamin A can work as either an antioxidant or prooxidant depending on its concentration. The present study was conducted to investigate the effects of different doses of vitamin A supplementation on the antioxidant system in rats.
MATERIALS/METHODS
Forty Spargue-Dawley male rats were divided into four groups according to the dose of vitamin A received: 0 (A0), 4,000 (A1), 8,000 (A2), and 20,000 (A3) IU retinyl palmitate/kg diet. After a feeding period of 4 wks, lipid peroxide levels, glutathione concentration, antioxidant enzyme activities, and vitamins A and E concentrations were measured. Histopathological changes were observed in rat liver tissue using an optical microscope and transmission electron microscope.
RESULTS
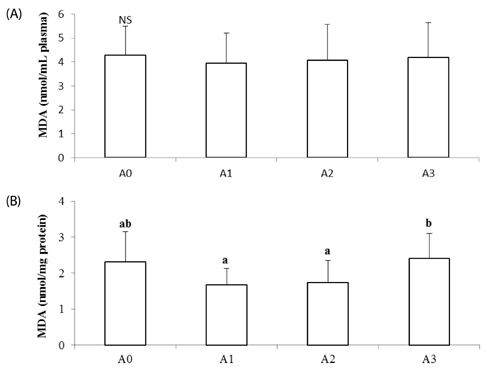
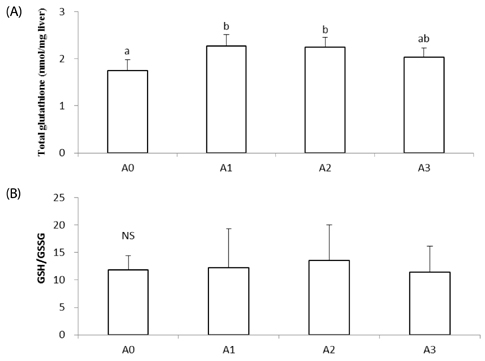
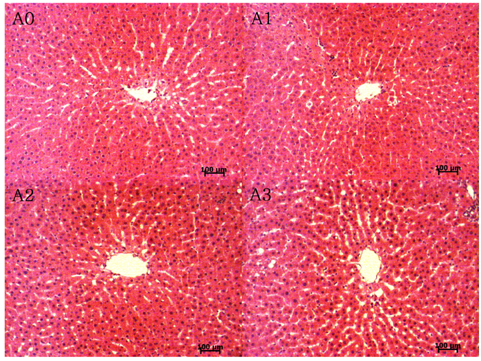
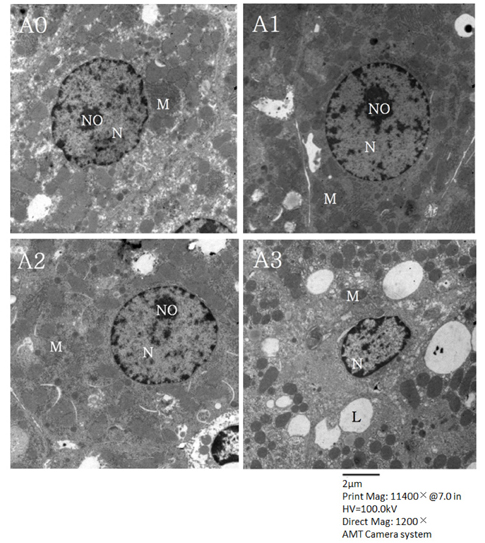
Lipid peroxide levels in plasma were significantly decreased in the A1 and A2 groups compared to the A0 rats. Erythrocyte catalase and hepatic superoxide dismutase activities of the A2 group were significantly higher than those of the A0 group. Hepatic glutathione peroxidase activity was significantly lower in the A3 group compared to the other groups. Total glutathione concentrations were significantly higher in the A1 and A2 groups than in the A0 group. Histological examination of liver tissue showed that excessive supplementation of vitamin A might lead to lipid droplet accumulation and nuclear membrane deformation.
CONCLUSIONS
These results indicate that appropriate supplementation of vitamin A might have a beneficial effect on the antioxidant system in rats.
MeSH Terms
Figure
Reference
-
1. Lee MJ, Kim JH. The changes of dietary reference intakes for Koreans and its application to the new text book. J Korean Home Econ Educ Assoc. 2008; 20:75–94.2. The Korean Nutrition Society. Dietary Reference Intakes for Koreans. 1st rev. ed. Seoul: The Korean Nutrition Society;2010.3. Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2010: Korea National Health and Nutrition Examination Survey (KNHANES V-1). Cheongwon: Korea Centers for Disease Control and Prevention;2011.4. Demissie T, Haider J, Tibeb HN, Haile B. Impact evaluation of EPI-PLUS and WIBS approaches in controlling vitamin A deficiency in Tigray and Harari regions, Ethiopia. Ethiop J Health Dev. 2000; 14:303–310.
Article5. Oliveros L, Vega V, Anzulovich AC, Ramirez D, Giménez MS. Vitamin A deficiency modifies antioxidant defenses and essential element contents in rat heart. Nutr Res. 2000; 20:1139–1150.
Article6. Vega VA, Anzulovich AC, Varas SM, Bonomi MR, Giménez MS, Oliveros LB. Effect of nutritional vitamin A deficiency on lipid metabolism in the rat heart: Its relation to PPAR gene expression. Nutrition. 2009; 25:828–838.
Article7. Tanumihardjo SA. Vitamin A and bone health: the balancing act. J Clin Densitom. 2013; 16:414–419.
Article8. Peluffo G, Radi R. Biochemistry of protein tyrosine nitration in cardiovascular pathology. Cardiovasc Res. 2007; 75:291–302.
Article9. Allen LH, Haskell M. Estimating the potential for vitamin A toxicity in women and young children. J Nutr. 2002; 132:2907S–2919S.
Article10. Binkley N, Krueger D. Hypervitaminosis A and bone. Nutr Rev. 2000; 58:138–144.
Article11. Pasquali MA, Gelain DP, Oliveira MR, Behr GA, Motta LL, Rocha RF, Klamt F, Moreira JC. Vitamin A supplementation induces oxidative stress and decreases the immunocontent of catalase and superoxide dismutase in rat lungs. Exp Lung Res. 2009; 35:427–438.
Article12. de Oliveira MR, Moreira JC. Acute and chronic vitamin A supplementation at therapeutic doses induces oxidative stress in submitochondrial particles isolated from cerebral cortex and cerebellum of adult rats. Toxicol Lett. 2007; 173:145–150.
Article13. McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969; 244:6049–6055.14. Hogeboom GH. Fractionation of cell components of animal tissues. Methods Enzymol. 1955; 1:16–19.15. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95:351–358.
Article16. Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976; 15:212–216.
Article17. Aebi H. Catalase. In : Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York (NY): Academic Press;1974. p. 673–683.18. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974; 47:469–474.
Article19. Pinto RE, Bartley W. The effect of age and sex on glutathione reductase and glutathione peroxidase activities and on aerobic glutathione oxidation in rat liver homogenates. Biochem J. 1969; 112:109–115.
Article20. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J Lab Clin Med. 1967; 70:158–169.21. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249:7130–7139.22. Bieri JG, Tolliver TJ, Catignani GL. Simultaneous determination of alpha-tocopherol and retinol in plasma or red cells by high pressure liquid chromatography. Am J Clin Nutr. 1979; 32:2143–2149.
Article23. Furr HC, Amédée-Manesme O, Olson JA. Gradient reversed-phase high-performance liquid chromatographic separation of naturally occurring retinoids. J Chromatogr. 1984; 309:299–307.
Article24. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Nonalcoholic Steatohepatitis Clinical Research Network. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005; 41:1313–1321.
Article25. Mori M. Electron microscopic and new microscopic studies of hepatocyte cytoskeleton: physiological and pathological relevance. J Electron Microsc (Tokyo). 1994; 43:347–355.26. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193:265–275.
Article27. Wedner SH, Ross DA. Vitamin A deficiency and its prevention. In : Heggenhougen K, Quah SR, editors. International Encyclopedia of Public Health. Amsterdam: Elsevier;2008. p. 526–532.28. Palace VP, Khaper N, Qin Q, Singal PK. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic Biol Med. 1999; 26:746–761.
Article29. Kim SC, Lee HJ, Joo JH, Yoon JH, Choi JY. Vitamin A deficiency induces fluid hyposecretion from the airway submucosal glands of mice. J Nutr. 2012; 142:739–743.
Article30. Arruda SF, Siqueira EM, de Valência FF. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition. 2009; 25:472–478.
Article31. Niki E. Do antioxidants impair signaling by reactive oxygen species and lipid oxidation products? FEBS Lett. 2012; 586:3767–3770.
Article32. de Oliveira MR, Soares Oliveira MW, Müller Hoff ML, Behr GA, da Rocha RF, Fonseca Moreira JC. Evaluation of redox and bioenergetics states in the liver of vitamin A-treated rats. Eur J Pharmacol. 2009; 610:99–105.
Article33. Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008; 29:9–16.
Article34. Lam HS, Chow CM, Poon WT, Lai CK, Chan KC, Yeung WL, Hui J, Chan AY, Ng PC. Risk of vitamin A toxicity from candy-like chewable vitamin supplements for children. Pediatrics. 2006; 118:820–824.
Article35. Vávrová A, Popelová O, Stěrba M, Jirkovský E, Hašková P, Mertlíková-Kaiserová H, Geršl V, Simůnek T. In vivo and in vitro assessment of the role of glutathione antioxidant system in anthracycline-induced cardiotoxicity. Arch Toxicol. 2011; 85:525–535.
Article36. Barber T, Borrás E, Torres L, García C, Cabezuelo F, Lloret A, Pallardó FV, Viña JR. Vitamin A deficiency causes oxidative damage to liver mitochondria in rats. Free Radic Biol Med. 2000; 29:1–7.
Article37. Gatica L, Alvarez S, Gomez N, Zago MP, Oteiza P, Oliveros L, Gimenez MS. Vitamin A deficiency induces prooxidant environment and inflammation in rat aorta. Free Radic Res. 2005; 39:621–628.
Article38. Esteban-Pretel G, Marín MP, Renau-Piqueras J, Barber T, Timoneda J. Vitamin A deficiency alters rat lung alveolar basement membrane: reversibility by retinoic acid. J Nutr Biochem. 2010; 21:227–236.
Article39. Elias-Miró M, Massip-Salcedo M, Raila J, Schweigert F, Mendes-Braz M, Ramalho F, Jiménez-Castro MB, Casillas-Ramírez A, Bermudo R, Rimola A, Rodes J, Peralta C. Retinol binding protein 4 and retinol in steatotic and nonsteatotic rat livers in the setting of partial hepatectomy under ischemia/reperfusion. Liver Transpl. 2012; 18:1198–1208.
Article40. Murakami K, Kaji T, Shimono R, Hayashida Y, Matsufuji H, Tsuyama S, Maezono R, Kosai K, Takamatsu H. Therapeutic effects of vitamin A on experimental cholestatic rats with hepatic fibrosis. Pediatr Surg Int. 2011; 27:863–870.
Article41. Aguilar RP, Genta S, Oliveros L, Anzulovich A, Giménez MS, Sánchez SS. Vitamin A deficiency injures liver parenchyma and alters the expression of hepatic extracellular matrix. J Appl Toxicol. 2009; 29:214–222.
Article42. Lenz AG, Costabel U, Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur Respir J. 1996; 9:307–312.
Article43. Klamt F, Roberto de Oliveira M, Moreira JC. Retinol induces permeability transition and cytochrome c release from rat liver mitochondria. Biochim Biophys Acta. 2005; 1726:14–20.
Article44. Loguercio C, De Girolamo V, de Sio I, Tuccillo C, Ascione A, Baldi F, Budillon G, Cimino L, Di Carlo A, Di Marino MP, Morisco F, Picciotto F, Terracciano L, Vecchione R, Verde V, Del Vecchio Blanco C. Non-alcoholic fatty liver disease in an area of southern Italy: main clinical, histological, and pathophysiological aspects. J Hepatol. 2001; 35:568–574.
Article45. Singal AK, Jampana SC, Weinman SA. Antioxidants as therapeutic agents for liver disease. Liver Int. 2011; 31:1432–1448.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Vitamin E Supplementation on Oxidative Stress in Streptozotocin Induced Diabetic Rats: Investigation of Liver and Plasma
- Effects of Antioxidant Supplementation on Lipid Profiles in Elderly Women
- Effects of P/S Ratio of Fatty Acids and Antioxidants Supplement on Serum Lipids Levels and Hepatic Antioxidants Enzyme Activities in Rats
- Retraction: Paper “Differential Effect of Vitamin K and Vitamin D Supplementation on Bone Mass in Young Rats Fed Normal or Low Calcium Diet†by Iwamoto J, et al. [Yonsei Med J 2004;45(2):314-324]
- The Effect of Alpha-tocopherol Supplementation on the Improvement of Antioxidant Status and Lymphocyte DNA Damage in Postmenopausal Women