Nutr Res Pract.
2013 Aug;7(4):287-293.
The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet
- Affiliations
-
- 1Department of Food Science and Nutrition, Dankook University, 126, Jukjeon-dong, Suji-gu, Yongin-si, Gyunggi 448-701, Korea. wkkim@dankook.ac.kr
Abstract
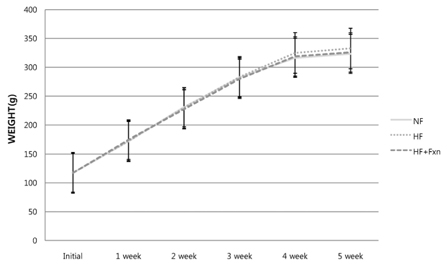
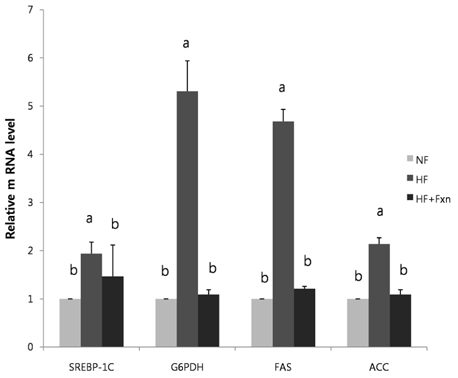
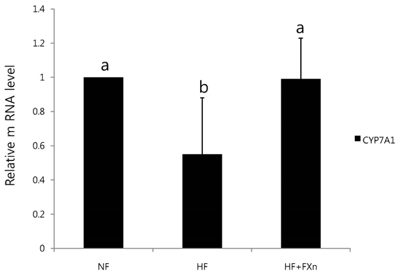
- This study determined the effects of fucoxanthin on gene expressions related to lipid metabolism in rats with a high-fat diet. Rats were fed with normal fat diet (NF, 7% fat) group, high fat diet group (HF, 20% fat), and high fat with 0.2% fucoxanthin diet group (HF+Fxn) for 4 weeks. Body weight changes and lipid profiles in plasma, liver, and feces were determined. The mRNA expressions of transcriptional factors such as sterol regulatory element binding protein (SREBP)-1c, Carnitine palmitoyltransferase-1 (CPT1), Cholesterol 7alpha-hydroxylase1 (CYP7A1) as well as mRNA expression of several lipogenic enzymes were determined. Fucoxanthin supplements significantly increased plasma high density lipoprotein (HDL) concentration (P < 0.05). The hepatic total lipids, total cholesterols, and triglycerides were significantly decreased while the fecal excretions of total lipids, cholesterol, and triglycerides were significantly increased in HF+Fxn group (P < 0.05). The mRNA expression of hepatic Acetyl-CoA carboxylase (ACC), Fatty acid synthase (FAS), and Glucose-6-phosphate dehydrogenase (G6PDH) as well as SREBP-1C were significantly lower in HF+Fxn group compared to the HF group (P < 0.05). The hepatic mRNA expression of Hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) and Acyl-CoA cholesterol acyltransferase (ACAT) were significantly low while lecithin-cholesterol acyltransferase (LCAT) was significantly high in the HF+Fxn group (P < 0.05). There was significant increase in mRNA expression of CPT1 and CYP7A1 in the HF+Fxn group, compared to the HF group (P < 0.05). In conclusion, consumption of fucoxanthin is thought to be effective in improving lipid and cholesterol metabolism in rats with a high fat diet.
Keyword
MeSH Terms
-
Acetyl-CoA Carboxylase
Animals
Body Weight Changes
Carnitine
Carrier Proteins
Cholesterol
Coenzyme A
Diet
Diet, High-Fat
Fatty Acid Synthetase Complex
Feces
Gene Expression
Glucosephosphate Dehydrogenase
Lipid Metabolism
Lipogenesis
Lipoproteins
Liver
Plasma
Rats
RNA, Messenger
Sterol O-Acyltransferase
Sterol Regulatory Element Binding Protein 1
Triglycerides
Xanthophylls
Acetyl-CoA Carboxylase
Carnitine
Carrier Proteins
Cholesterol
Coenzyme A
Fatty Acid Synthetase Complex
Glucosephosphate Dehydrogenase
Lipoproteins
RNA, Messenger
Sterol O-Acyltransferase
Sterol Regulatory Element Binding Protein 1
Triglycerides
Xanthophylls
Figure
Reference
-
1. Barres R, Zierath JR. DNA methylation in metabolic disorders. Am J Clin Nutr. 2011; 93:897S–900S.
Article2. Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005; 16:421–427.
Article3. Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005; 42:44–52.
Article4. MacArtain P, Gill CI, Brooks M, Campbell R, Rowland IR. Nutritional value of edible seaweeds. Nutr Rev. 2007; 65:535–543.
Article5. Chandini SK, Ganesan P, Suresh PV, Bhaskar N. Seaweeds as a source of nutritionally beneficial compounds - a review. J Food Sci Technol. 2008; 45:1–3.6. Maeda H, Hosokawa M, Sashima T, Funayama K, Miyashita K. Fucoxanthin from edible seaweed, Undaria pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem Biophys Res Commun. 2005; 332:392–397.
Article7. Miyashita K, Maeda H, Okada T, Abe M, Hosokawa M. Anti-obesity and anti-diabetic effects of allenic carotenoid, fucoxanthin. Agro Food Ind Hi Tech. 2010; 21:24–27.8. Okada T, Nakai M, Maeda H, Hosokawa M, Sashima T, Miyashita K. Suppressive effect of neoxanthin on the differentiation of 3T3-L1 adipose cells. J Oleo Sci. 2008; 57:345–351.
Article9. Yim MJ, Hosokawa M, Mizushina Y, Yoshida H, Saito Y, Miyashita K. Suppressive effects of amarouciaxanthin A on 3T3-L1 adipocyte differentiation through down-regulation of PPARγ and C/EBPα mRNA expression. J Agric Food Chem. 2011; 59:1646–1652.
Article10. Park HJ, Lee MK, Park YB, Shin YC, Choi MS. Beneficial effects of Undaria pinnatifida ethanol extract on diet-induced-insulin resistance in C57BL/6J mice. Food Chem Toxicol. 2011; 49:727–733.
Article11. Maeda H, Hosokawa M, Sashima T, Miyashita K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK-Ay mice. J Agric Food Chem. 2007; 55:7701–7706.
Article12. Woo MN, Jeon SM, Shin YC, Lee MK, Kang MA, Choi MS. Anti-obese property of fucoxanthin is partly mediated by altering lipid-regulating enzymes and uncoupling proteins of visceral adipose tissue in mice. Mol Nutr Food Res. 2009; 53:1603–1611.
Article13. Jeon SM, Kim HJ, Woo MN, Lee MK, Shin YC, Park YB, Choi MS. Fucoxanthin-rich seaweed extract suppresses body weight gain and improves lipid metabolism in high-fat-fed C57BL/6J mice. Biotechnol J. 2010; 5:961–969.
Article14. Maeda H, Hosokawa M, Sashima T, Murakami-Funayama K, Miyashita K. Anti-obesity and anti-diabetic effects of fucoxanthin on diet-induced obesity conditions in a murine model. Mol Med Rep. 2009; 2:897–902.
Article15. Hu X, Li Y, Li C, Fu Y, Cai F, Chen Q, Li D. Combination of fucoxanthin and conjugated linoleic acid attenuates body weight gain and improves lipid metabolism in high-fat diet-induced obese rats. Arch Biochem Biophys. 2012; 519:59–65.
Article16. Woo MN, Jeon SM, Kim HJ, Lee MK, Shin SK, Shin YC, Park YB, Choi MS. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem Biol Interact. 2010; 186:316–322.
Article17. Mayes PA. Biosynthesis of fatty acids. In : Murray RK, Harper HA, editors. Harper's Biochemistry. 25th ed. Stamford (CT): Appleton and Lange;2000. p. 230–257.18. Chen J, Zhao H, Ma X, Han X, Luo L, Wang L, Han J, Liu B, Wang W. The effects of jiang-zhi-ning and its main components on cholesterol metabolism. Evid Based Complement Alternat Med. 2012; 2012:928234.
Article19. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001; 25:402–408.
Article20. Frings CS, Dunn RT. A colorimetric method for determination of total serum lipids based on the sulfo-phospho-vanillin reaction. Am J Clin Pathol. 1970; 53:89–91.
Article21. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957; 226:497–509.
Article22. Bettzieche A, Brandsch C, Hirche F, Eder K, Stangl GI. L-cysteine down-regulates SREBP-1c-regulated lipogenic enzymes expression via glutathione in HepG2 cells. Ann Nutr Metab. 2008; 52:196–203.
Article23. Foretz M, Guichard C, Ferré P, Foufelle F. Sterol regulatory element binding protein-1c is a major mediator of insulin action on the hepatic expression of glucokinase and lipogenesis-related genes. Proc Natl Acad Sci U S A. 1999; 96:12737–12742.
Article24. Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997; 89:331–340.
Article25. Shimomura I, Hammer RE, Richardson JA, Ikemoto S, Bashmakov Y, Goldstein JL, Brown MS. Insulin resistance and diabetes mellitus in transgenic mice expressing nuclear SREBP-1c in adipose tissue: model for congenital generalized lipodystrophy. Genes Dev. 1998; 12:3182–3194.
Article26. Diraison F, Dusserre E, Vidal H, Sothier M, Beylot M. Increased hepatic lipogenesis but decreased expression of lipogenic gene in adipose tissue in human obesity. Am J Physiol Endocrinol Metab. 2002; 282:E46–E51.27. Nicolle C, Cardinault N, Aprikian O, Busserolles J, Grolier P, Rock E, Demigné C, Mazur A, Scalbert A, Amouroux P, Rémésy C. Effect of carrot intake on cholesterol metabolism and on antioxidant status in cholesterol-fed rat. Eur J Nutr. 2003; 42:254–261.
Article28. Matsumoto M, Hosokawa M, Matsukawa N, Hagio M, Shinoki A, Nishimukai M, Miyashita K, Yajima T, Hara H. Suppressive effects of the marine carotenoids, fucoxanthin and fucoxanthinol on triglyceride absorption in lymph duct-cannulated rats. Eur J Nutr. 2010; 49:243–249.
Article29. Siperstein MD. Regulation of cholesterol biosynthesis in normal and malignant tissues. Curr Top Cell Regul. 1970; 2:65–100.30. Pullinger CR, Eng C, Salen G, Shefer S, Batta AK, Erickson SK, Verhagen A, Rivera CR, Mulvihill SJ, Malloy MJ, Kane JP. Human cholesterol 7alpha-hydroxylase (CYP7A1) deficiency has a hypercholesterolemic phenotype. J Clin Invest. 2002; 110:109–117.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antioxidant effects of fucoxanthin rich powder in rats fed with high fat diet
- Effect of Chlorella vulgaris on lipid metabolism in Wistar rats fed high fat diet
- Effects of Soyoligosaccharide on Lipid Metabolism in Rats Fed the High Fat or Low Fat Diet
- Effects of Genistein Supplementation on Fatty Liver and Lipid Metabolism in Rats Fed High Fat Diet
- Effect of Dietary Grape Pomace on Lipid Oxidation and Related Enzyme Activities in Rats Fed High Fat Diet