Nutr Res Pract.
2012 Oct;6(5):405-413.
Optimized mixture of hops rho iso-alpha acids-rich extract and acacia proanthocyanidins-rich extract reduces insulin resistance in 3T3-L1 adipocytes and improves glucose and insulin control in db/db mice
- Affiliations
-
- 1MetaProteomics LLC, 9770 44 Ave. NW, Ste 100, Gig Harbor, WA 98332, USA. matthewtripp@metagenics.com
- 2Bionexus, Ltd, 30 Brown Road, Ithaca, NY 14850, USA.
Abstract
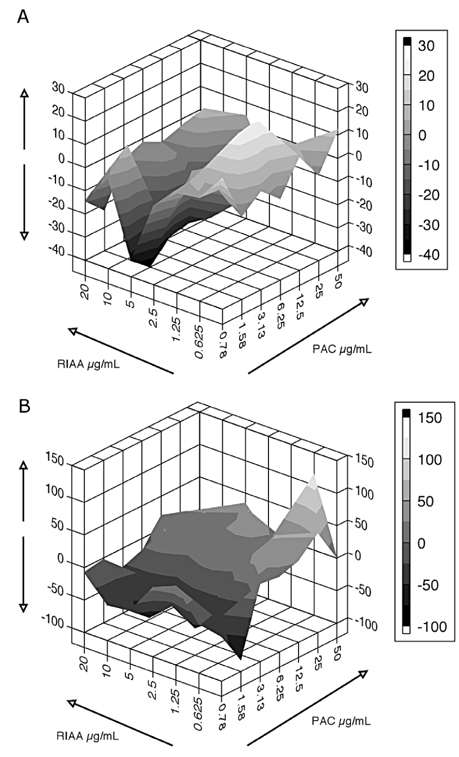
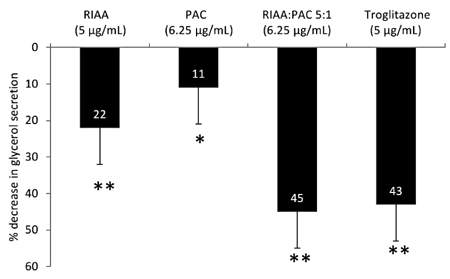
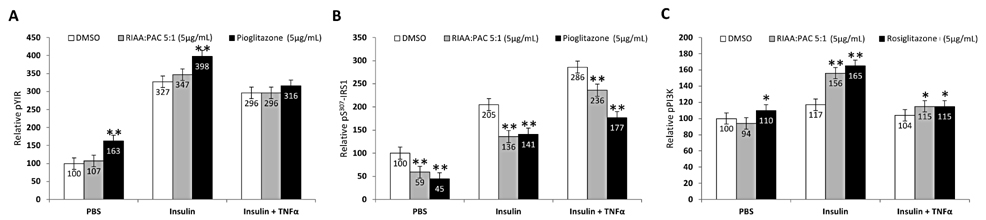
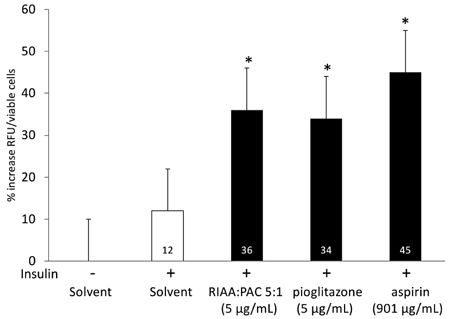
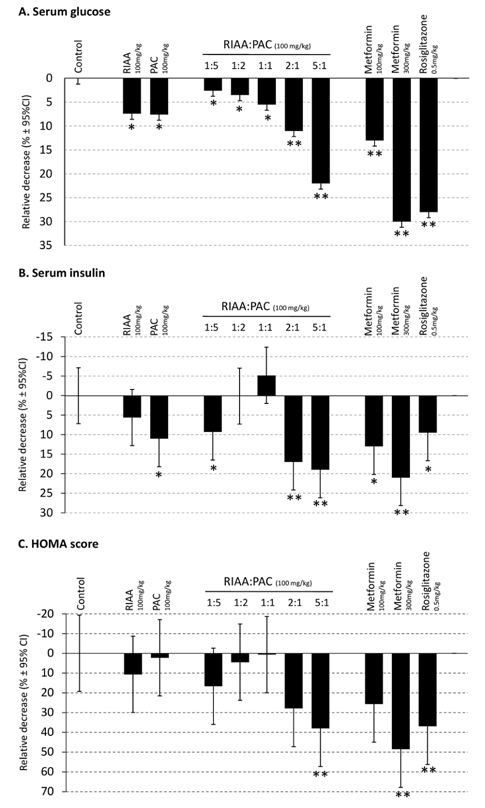
- Rho iso-alpha acids-rich extract (RIAA) from Humulus lupulus (hops) and proanthocyanidins-rich extracts (PAC) from Acacia nilotica exert anti-inflammatory and anti-diabetic activity in vitro and in vivo. We hypothesized that a combination of these two extracts would exert enhanced effects in vitro on inflammatory markers and insulin signaling, and on nonfasting glucose and insulin in db/db mice. Over 49 tested combinations, RIAA:PAC at 5:1 (6.25 microg/mL) exhibited the greatest reductions in TNFalpha-stimulated lipolysis and IL-6 release in 3T3-L1 adipocytes, comparable to 5 microg/mL troglitazone. Pretreatment of 3T3-L1 adipocytes with this combination (5 microg/mL) also led to a 3-fold increase in insulin-stimulated glucose uptake that was comparable to 5 microg/mL pioglitazone or 901 microg/mL aspirin. Finally, db/db mice fed with RIAA:PAC at 5:1 (100 mg/kg) for 7 days resulted in 22% decrease in nonfasting glucose and 19% decrease in insulin that was comparable to 0.5 mg/kg rosiglitazone and better than 100 mg/kg metformin. RIAA:PAC mixture may have the potential to be an alternative when conventional therapy is undesirable or ineffective, and future research exploring its long-term clinical application is warranted.
MeSH Terms
Figure
Reference
-
1. Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S. Diabetes Care. 2005. 28:2745–2749.
Article2. Alexander CM. The coming of age of the metabolic syndrome. Diabetes Care. 2003. 26:3180–3181.
Article3. Isomaa B, Henricsson M, Almgren P, Tuomi T, Taskinen MR, Groop L. The metabolic syndrome influences the risk of chronic complications in patients with type II diabetes. Diabetologia. 2001. 44:1148–1154.
Article4. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991. 14:173–194.
Article5. Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003. 112:1821–1830.
Article6. Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008. 28:1654–1659.
Article7. Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007. 56:1010–1013.
Article8. Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995. 95:2409–2415.
Article9. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002. 51:7–18.10. Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signalling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006. 7:85–96.
Article11. Kirpichnikov D, McFarlane SI, Sowers JR. Metformin: an update. Ann Intern Med. 2002. 137:25–33.
Article12. Bailey CJ. Treating insulin resistance in type 2 diabetes with metformin and thiazolidinediones. Diabetes Obes Metab. 2005. 7:675–691.
Article13. Doggrell SA. Clinical trials with thiazolidinediones in subjects with Type 2 diabetes--is pioglitazone any different from rosiglitazone? Expert Opin Pharmacother. 2008. 9:405–420.
Article14. Amiel SA, Dixon T, Mann R, Jameson K. Hypoglycaemia in Type 2 diabetes. Diabet Med. 2008. 25:245–254.
Article15. Hernandez AV, Usmani A, Rajamanickam A, Moheet A. Thiazolidinediones and risk of heart failure in patients with or at high risk of type 2 diabetes mellitus: a meta-analysis and meta-regression analysis of placebo-controlled randomized clinical trials. Am J Cardiovasc Drugs. 2011. 11:115–128.
Article16. Babish JG, Pacioretty LM, Bland JS, Minich DM, Hu J, Tripp ML. Antidiabetic screening of commercial botanical products in 3T3-L1 adipocytes and db/db mice. J Med Food. 2010. 13:535–547.
Article17. Konda VR, Desai A, Darland G, Bland JS, Tripp ML. Rho iso-alpha acids from hops inhibit the GSK-3/NF-kappaB pathway and reduce inflammatory markers associated with bone and cartilage degradation. J Inflamm (Lond). 2009. 6:26.
Article18. Lerman RH, Minich DM, Darland G, Lamb JJ, Schiltz B, Babish JG, Bland JS, Tripp ML. Enhancement of a modified Mediterranean-style, low glycemic load diet with specific phytochemicals improves cardiometabolic risk factors in subjects with metabolic syndrome and hypercholesterolemia in a randomized trial. Nutr Metab (Lond). 2008. 5:29.
Article19. Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953. 3:285–290.20. Yeh P, Kishony R. Networks from drug-drug surfaces. Mol Syst Biol. 2007. 3:85.
Article21. Flick DA, Gifford GE. Comparison of in vitro cell cytotoxic assays for tumor necrosis factor. J Immunol Methods. 1984. 68:167–175.
Article22. Leira F, Louzao MC, Vieites JM, Botana LM, Vieytes MR. Fluorescent microplate cell assay to measure uptake and metabolism of glucose in normal human lung fibroblasts. Toxicol In Vitro. 2002. 16:267–273.
Article23. Burr JF, Rowan CP, Jamnik VK, Riddell MC. The role of physical activity in type 2 diabetes prevention: physiological and practical perspectives. Phys Sportsmed. 2010. 38:72–82.
Article24. Esposito K, Giugliano D. Mediterranean diet and the metabolic syndrome: the end of the beginning. Metab Syndr Relat Disord. 2010. 8:197–200.
Article25. Good CB. Polypharmacy in elderly patients with diabetes. Diabetes Spectr. 2002. 15:240–248.
Article26. Turner RC, Cull CA, Frighi V, Holman RR. UK Prospective Diabetes Study (UKPDS) Group. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA. 1999. 281:2005–2012.
Article27. Minich DM, Lerman RH, Darland G, Babish JG, Pacioretty LM, Bland JS, Tripp ML. Hop and Acacia Phytochemicals Decreased Lipotoxicity in 3T3-L1 Adipocytes, db/db Mice, and Individuals with Metabolic Syndrome. J Nutr Metab. 2010. 2010:pii: 467316.28. Arner P. Not all fat is alike. Lancet. 1998. 351:1301–1302.
Article29. Bergman RN, Kim SP, Hsu IR, Catalano KJ, Chiu JD, Kabir M, Richey JM, Ader M. Abdominal obesity: role in the pathophysiology of metabolic disease and cardiovascular risk. Am J Med. 2007. 120:S3–S8. discussion S29-32.
Article30. Kabir M, Catalano KJ, Ananthnarayan S, Kim SP, Van Citters GW, Dea MK, Bergman RN. Molecular evidence supporting the portal theory: a causative link between visceral adiposity and hepatic insulin resistance. Am J Physiol Endocrinol Metab. 2005. 288:E454–E461.
Article31. Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011. 60:56–63.
Article32. Bajaj M, Suraamornkul S, Romanelli A, Cline GW, Mandarino LJ, Shulman GI, DeFronzo RA. Effect of a sustained reduction in plasma free fatty acid concentration on intramuscular long-chain fatty Acyl-CoAs and insulin action in type 2 diabetic patients. Diabetes. 2005. 54:3148–3153.
Article33. Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000. 106:171–176.
Article34. Hotamisligil GS, Budavari A, Murray D, Spiegelman BM. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J Clin Invest. 1994. 94:1543–1549.
Article35. Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996. 271:665–668.
Article36. Standaert ML, Bandyopadhyay G, Kanoh Y, Sajan MP, Farese RV. Insulin and PIP3 activate PKC-zeta by mechanisms that are both dependent and independent of phosphorylation of activation loop (T410) and autophosphorylation (T560) sites. Biochemistry. 2001. 40:249–255.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sargassum coreanum extract alleviates hyperglycemia and improves insulin resistance in db/db diabetic mice
- Nelumbo nucifera Leaf Extract Regulates Lipid Metabolism and Differentiation in 3T3-L1 Adipocytes and db/db Mice
- Gynura procumbens extract improves insulin sensitivity and suppresses hepatic gluconeogenesis in C57BL/KsJ-db/db mice
- Daraesoon (shoot of hardy kiwi) mitigates hyperglycemia in db/db mice by alleviating insulin resistance and inflammation
- Pear pomace ethanol extract improves insulin resistance through enhancement of insulin signaling pathway without lipid accumulation