Nutr Res Pract.
2017 Jun;11(3):198-205. 10.4162/nrp.2017.11.3.198.
Pear pomace ethanol extract improves insulin resistance through enhancement of insulin signaling pathway without lipid accumulation
- Affiliations
-
- 1Department of Food and Nutrition / Research Institute of Human Ecology, Mokpo National University, 1666, Yeongsan-ro, Cheonggye-myeon, Muan-gun, Jeonam 58554, Korea. kha@mokpo.ac.kr
- KMID: 2407767
- DOI: http://doi.org/10.4162/nrp.2017.11.3.198
Abstract
- BACKGROUND/OBJECTIVES
The anti-diabetic activity of pear through inhibition of α-glucosidase has been demonstrated. However, little has been reported about the effect of pear on insulin signaling pathway in obesity. The aims of this study are to establish pear pomace 50% ethanol extract (PPE)-induced improvement of insulin sensitivity and characterize its action mechanism in 3T3-L1 cells and high-fat diet (HFD)-fed C57BL/6 mice.
MATERIALS/METHODS
Lipid accumulation, monocyte chemoattractant protein-1 (MCP-1) secretion and glucose uptake were measure in 3T3-L1 cells. Mice were fed HFD (60% kcal from fat) and orally ingested PPE once daily for 8 weeks and body weight, homeostasis model assessment of insulin resistance (HOMA-IR), and serum lipids were measured. The expression of proteins involved in insulin signaling pathway was evaluated by western blot assay in 3T3-L1 cells and adipose tissue of mice.
RESULTS
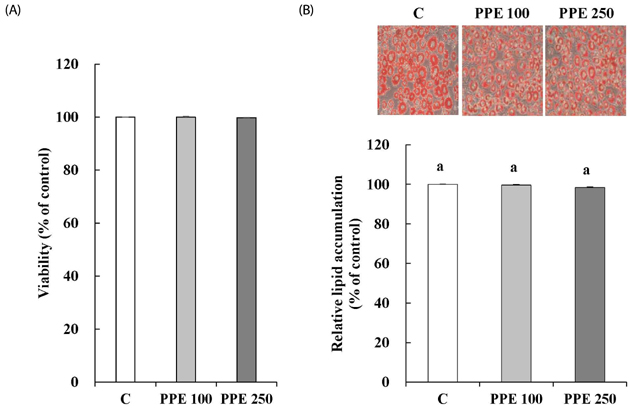
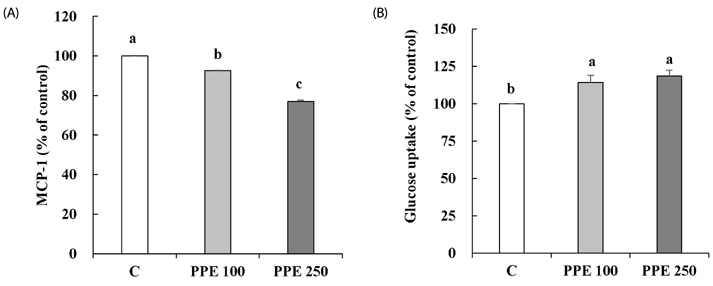
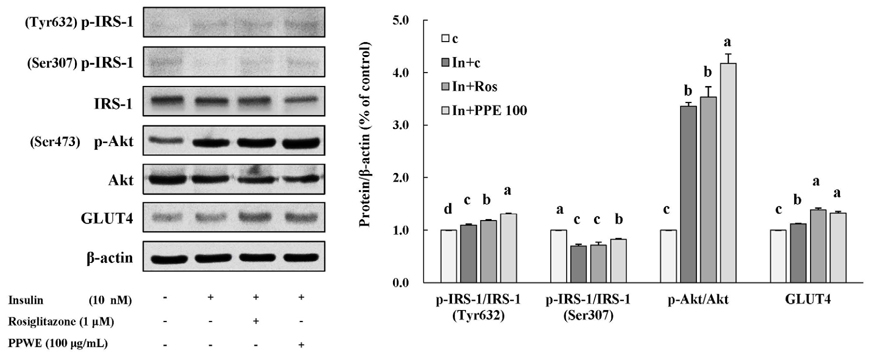
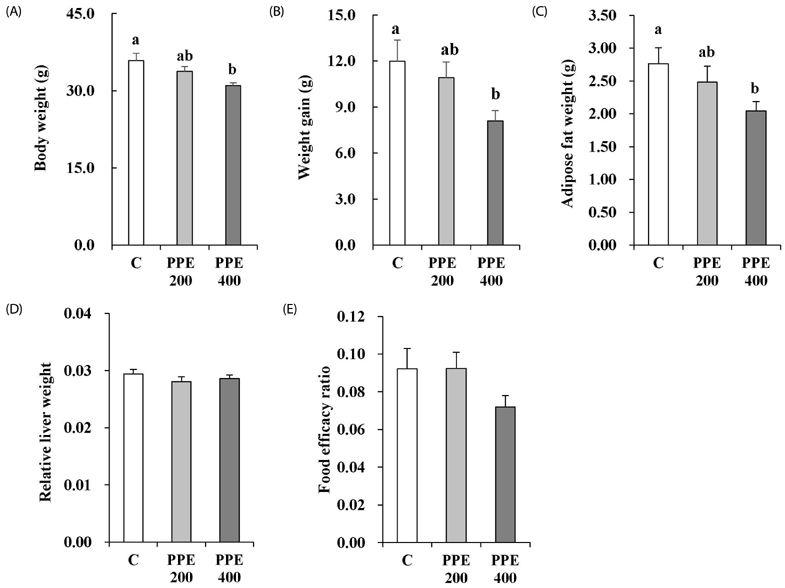
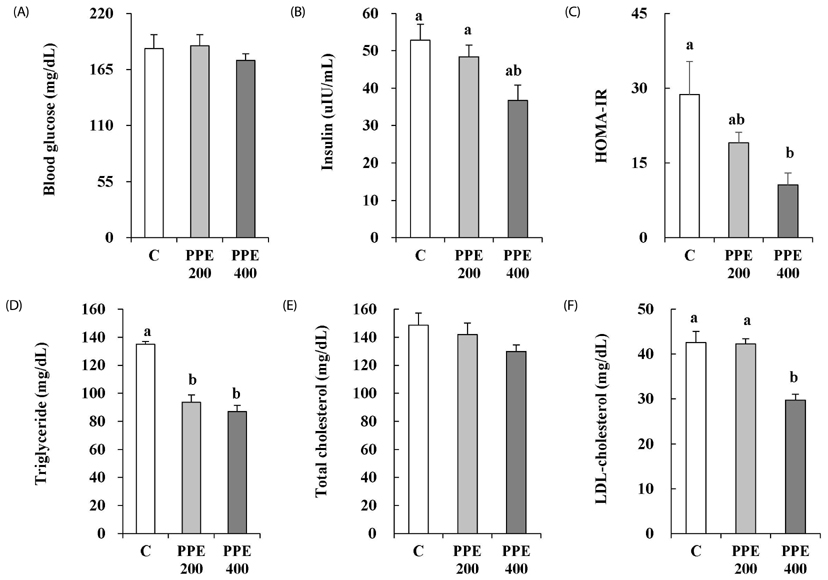
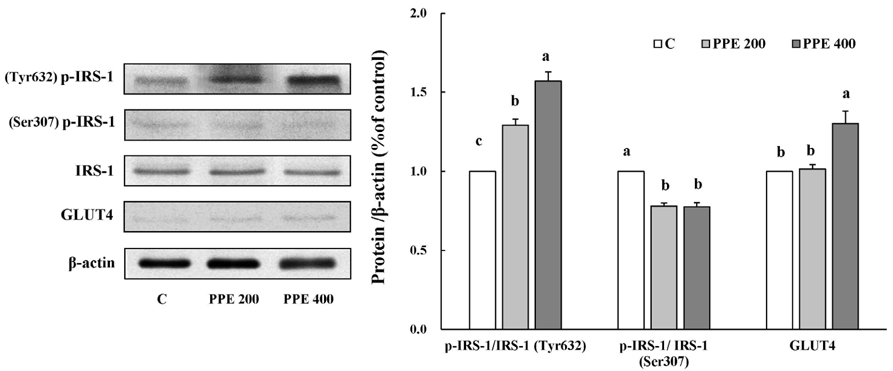
In 3T3-L1 cells, without affecting cell viability and lipid accumulation, PPE inhibited MCP-1 secretion, improved glucose uptake, and increased protein expression of phosphorylated insulin receptor substrate 1 [p-IRS-1, (Tyrⶳ²)], p-Akt, and glucose transporter type 4 (GLUT4). Additionally, in HFD-fed mice, PPE reduced body weight, HOMA-IR, and serum lipids including triglyceride and LDL-cholesterol. Furthermore, in adipose tissue, PPE up-regulated GLUT4 expression and expression ratio of p-IRS-1 (Tyrⶳ²)/IRS, whereas, down-regulated p-IRS-1 (Ser³â°â·)/IRS.
CONCLUSIONS
Our results collectively show that PPE improves glucose uptake in 3T3-L1 cells and insulin sensitivity in mice fed a HFD through stimulation of the insulin signaling pathway. Furthermore, PPE-induced improvement of insulin sensitivity was not accompanied with lipid accumulation.
MeSH Terms
-
3T3-L1 Cells
Adipose Tissue
Animals
Blotting, Western
Body Weight
Cell Survival
Chemokine CCL2
Diet, High-Fat
Ethanol*
Glucose
Glucose Transport Proteins, Facilitative
Glucose Transporter Type 4
Homeostasis
Insulin Receptor Substrate Proteins
Insulin Resistance*
Insulin*
Lipid Metabolism
Mice
Obesity
Pyrus*
Triglycerides
Chemokine CCL2
Ethanol
Glucose
Glucose Transport Proteins, Facilitative
Glucose Transporter Type 4
Insulin
Insulin Receptor Substrate Proteins
Figure
Cited by 2 articles
-
Reactive oxygen species-dependent apoptosis induction by water extract of Citrus unshiu peel in MDA-MB-231 human breast carcinoma cells
Min Yeong Kim, Eun Ok Choi, Hyun HwangBo, Da He Kwon, Kyu Im Ahn, Hong Jae Kim, Seon Yeong Ji, Su-Hyun Hong, Jin-Woo Jeong, Gi Young Kim, Cheol Park, Yung Hyun Choi
Nutr Res Pract. 2018;12(2):129-134. doi: 10.4162/nrp.2018.12.2.129.Pear pomace alleviated atopic dermatitis in NC/Nga mice and inhibited LPS-induced inflammation in RAW 264.7 macrophages
Mikyoung You, Ziyun Wang, Hwa-Jin Kim, Young-Hyun Lee, Hyeon-A Kim
Nutr Res Pract. 2022;16(5):577-588. doi: 10.4162/nrp.2022.16.5.577.
Reference
-
1. Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006; 444:840–846.
Article2. Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006; 68:123–158.
Article3. Pessin JE, Saltiel AR. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest. 2000; 106:165–169.
Article4. Saad MJ, Araki E, Miralpeix M, Rothenberg PL, White MF, Kahn CR. Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest. 1992; 90:1839–1849.
Article5. Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011; 12:722–734.
Article6. Rajala MW, Scherer PE. Minireview: the adipocyte--at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003; 144:3765–3773.
Article7. Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008; 9:367–377.
Article8. White MF. Insulin signaling in health and disease. Science. 2003; 302:1710–1711.
Article9. Pilch PF, Lee J. Insulin receptor family. In : Lennarz WJ, Lane MD, editors. Encyclopedia of Biological Chemistry. Vol. 2. Oxford: Elsevier Academic Press;2004. p. 436–440.10. Muretta JM, Mastick CC. How insulin regulates glucose transport in adipocytes. Vitam Horm. 2009; 80:245–286.11. Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007; 5:237–252.
Article12. Xie L, Ortega MT, Mora S, Chapes SK. Interactive changes between macrophages and adipocytes. Clin Vaccine Immunol. 2010; 17:651–659.
Article13. Kahn SE, Cooper ME, Del Prato S. Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future. Lancet. 2014; 383:1068–1083.
Article14. Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995; 270:12953–12956.
Article15. Diamant M, Heine RJ. Thiazolidinediones in type 2 diabetes mellitus: current clinical evidence. Drugs. 2003; 63:1373–1405.16. Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. American Heart Association. American Diabetes Association. Thiazolidinedione use, fluid retention, and congestive heart failure: a consensus statement from the American Heart Association and American Diabetes Association. October 7, 2003. Circulation. 2003; 108:2941–2948.
Article17. Bazelier MT, Gallagher AM, van Staa TP, Cooper C, Leufkens HG, Vestergaard P, de Vries F. Use of thiazolidinediones and risk of osteoporotic fracture: disease or drugs? Pharmacoepidemiol Drug Saf. 2012; 21:507–514.
Article18. Chang CL, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies for type 2 diabetes mellitus: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med. 2013; 2013:378657.
Article19. Singh R, Kaur N, Kishore L, Gupta GK. Management of diabetic complications: a chemical constituents based approach. J Ethnopharmacol. 2013; 150:51–70.
Article20. Rabetafika HN, Bchir B, Blecker C, Paquot M, Wathelet B. Comparative study of alkaline extraction process of hemicelluloses from pear pomace. Biomass Bioenergy. 2014; 61:254–264.
Article21. Li X, Wang T, Zhou B, Gao W, Cao J, Huang L. Chemical composition and antioxidant and anti-inflammatory potential of peels and flesh from 10 different pear varieties (Pyrus spp.). Food Chem. 2014; 152:531–538.
Article22. Boath AS, Stewart D, McDougall GJ. Berry components inhibit α-glucosidase in vitro: synergies between acarbose and polyphenols from black currant and rowanberry. Food Chem. 2012; 135:929–936.
Article23. Oboh G, Ademiluyi AO, Akinyemi AJ, Henle T, Saliu JA, Schwarzenbolz U. Inhibitory effect of polyphenol-rich extracts of jute leaf (Corchorus olitorius) on key enzyme linked to type-2 diabetes (α-amylase and α-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Foods. 2012; 4:450–458.
Article24. Zhang L, Hogan S, Li J, Sun S, Canning C, Zheng SJ, Zhou K. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011; 126:466–471.
Article25. Wang T, Li X, Zhou B, Li H, Zeng J, Gao W. Anti-diabetic activity in type 2 diabetic mice and α-glucosidase inhibitory, antioxidant and anti-inflammatory potential of chemically profiled pear peel and pulp extracts (Pyrus spp.). J Funct Foods. 2015; 13:276–288.
Article26. Kim J, Park Y, Yoon KS, Clark JM, Park Y. Permethrin alters adipogenesis in 3T3-L1 adipocytes and causes insulin resistance in C2C12 myotubes. J Biochem Mol Toxicol. 2014; 28:418–424.
Article27. Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004; 24:29–33.
Article28. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985; 28:412–419.
Article29. Fernández-Veledo S, Nieto-Vazquez I, Vila-Bedmar R, Garcia-Guerra L, Alonso-Chamorro M, Lorenzo M. Molecular mechanisms involved in obesity-associated insulin resistance: therapeutical approach. Arch Physiol Biochem. 2009; 115:227–239.
Article30. James DE, Brown R, Navarro J, Pilch PF. Insulin-regulatable tissues express a unique insulin-sensitive glucose transport protein. Nature. 1988; 333:183–185.
Article31. Zhou L, Yang Y, Wang X, Liu S, Shang W, Yuan G, Li F, Tang J, Chen M, Chen J. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007; 56:405–412.
Article32. Garvey WT, Maianu L, Huecksteadt TP, Birnbaum MJ, Molina JM, Ciaraldi TP. Pretranslational suppression of a glucose transporter protein causes insulin resistance in adipocytes from patients with non-insulin-dependent diabetes mellitus and obesity. J Clin Invest. 1991; 87:1072–1081.
Article33. Kahn BB, Flier JS. Obesity and insulin resistance. J Clin Invest. 2000; 106:473–481.
Article34. Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring). 2007; 15:798–808.
Article35. Song H, Han W, Yan F, Xu D, Chu Q, Zheng X. Dietary Phaseolus vulgaris extract alleviated diet-induced obesity, insulin resistance and hepatic steatosis and alters gut microbiota composition in mice. J Funct Foods. 2016; 20:236–244.
Article36. Fraulob JC, Ogg-Diamantino R, Fernandes-Santos C, Aguila MB, Mandarim-de-Lacerda CA. A mouse model of metabolic syndrome: Insulin resistance, fatty liver and non-alcoholic fatty pancreas disease (NAFPD) in C57BL/6 mice fed a high fat diet. J Clin Biochem Nutr. 2010; 46:212–223.
Article37. Tsai CW, Liu KL, Lin YR, Kuo WC. The mechanisms of carnosic acid attenuates tumor necrosis factor-α-mediated inflammation and insulin resistance in 3T3-L1 adipocytes. Mol Nutr Food Res. 2014; 58:654–664.
Article38. Hwang JS, Park JW, Nam MS, Cho H, Han IO. Glucosamine enhances body weight gain and reduces insulin response in mice fed chow diet but mitigates obesity, insulin resistance and impaired glucose tolerance in mice high-fat diet. Metabolism. 2015; 64:368–379.
Article39. Williams VL, Martin RE, Franklin JL, Hardy RW, Messina JL. Injury-induced insulin resistance in adipose tissue. Biochem Biophys Res Commun. 2012; 421:442–448.
Article40. Araújo TG, de Oliveira AG, Vecina JF, Marin RM, Franco ES, Abdalla Saad MJ, de Sousa Maia MB. Parkinsonia aculeata (Caesalpineaceae) improves high-fat diet-induced insulin resistance in mice through the enhancement of insulin signaling and mitochondrial biogenesis. J Ethnopharmacol. 2016; 183:95–102.
Article41. Yamashita H, Kusudo T, Takeuchi T, Qiao S, Tsutsumiuchi K, Wang T, Wang Y. Dietary supplementation with evodiamine prevents obesity and improves insulin resistance in ageing mice. J Funct Foods. 2015; 19:320–329.
Article