Lab Med Online.
2015 Apr;5(2):92-100. 10.3343/lmo.2015.5.2.92.
A Comparison of the Performance of Soyagreentec Ampulab EDTA and Sodium Citrate Tubes with That of BD Vacutainer Tubes
- Affiliations
-
- 1Department of Laboratory Medicine, Chung-Ang University College of Medicine, Seoul, Korea. chayoung@cau.ac.kr
- 2Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2312281
- DOI: http://doi.org/10.3343/lmo.2015.5.2.92
Abstract
- BACKGROUND
Because blood collection tube can affect the results of various laboratory tests, it is necessary before using a newly developed product to verify its performance stringently and objectively. We compared the performance of Ampulab EDTA and sodium citrate tubes (Soyagreentec, Korea) with that of Vacutainer tubes (BD, USA) in accordance with international guidelines.
METHODS
This study was performed as a multicenter study of Chung-Ang University Hospital and Seoul National University Hospital to evaluate the performance of two different instrument platforms. We performed the precision test according to CLSI GP34-A, the accuracy test according to CLSI EP09-A2-IR, and the vacuum test according to CLSI H1-A5 as well as stability, and aseptic condition tests. We evaluated 3 lots of Ampulab tubes for their precision, accuracy, vacuum, and aseptic condition.
RESULTS
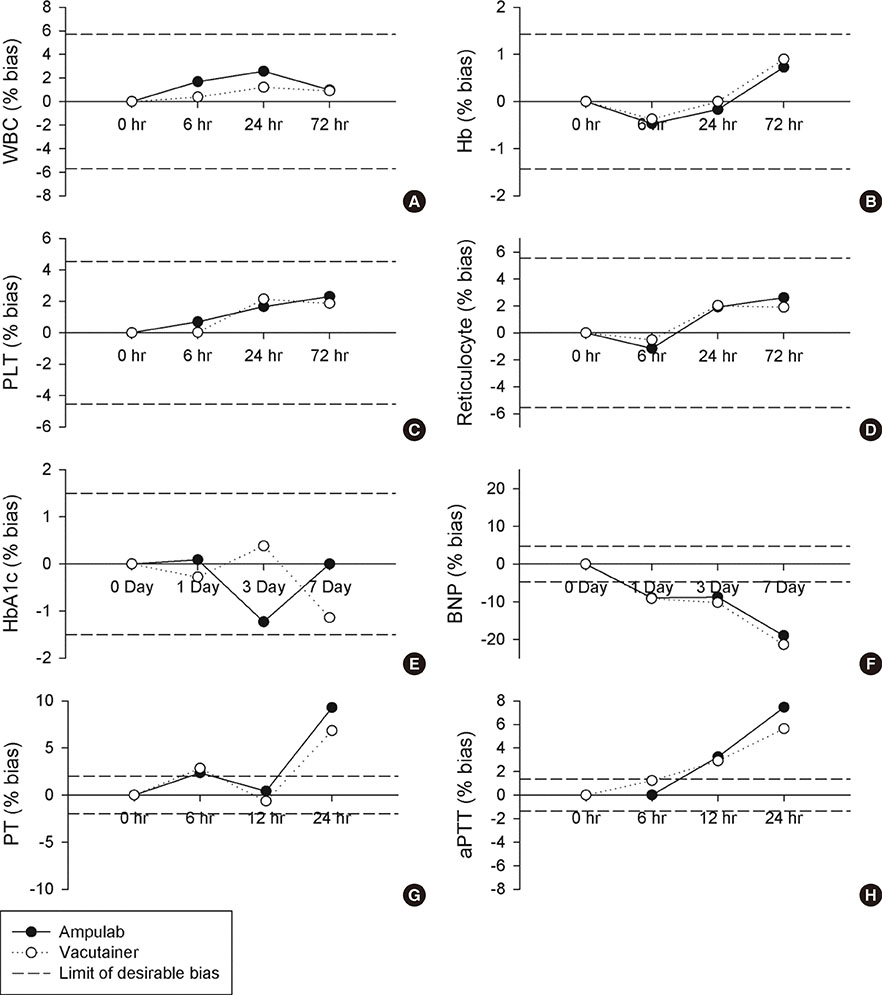
In precision test, the total precision levels of Ampulab tubes in most measurands were desirable or allowable. The results of mean corpuscular hemoglobin concentration, platelet distribution width, basophil, and reticulocyte counts for Ampulab tubes showed imprecision beyond allowable limits, but were similar to those of Vacutainer tubes. In the accuracy test, the bias in most measurands, except for the mean platelet volume, was within allowable limits. In the stability test, Ampulab showed similar performance to Vacutainer. In tests of the vacuum and aseptic conditions, Ampulab fulfilled both requirements.
CONCLUSIONS
The performance of Ampulab EDTA and sodium citrate tubes was equivalent to that of Vacutainer tubes.
Keyword
MeSH Terms
Figure
Reference
-
1. Oh SH, Kim CS. [Comparison of two new plastic tubes (Sekisui INSEPACK and Green Cross Green Vac-Tube) with BD Vacutainer tubes for 49 analytes]. Korean J Lab Med. 2007; 27:69–75.
Article2. Won EJ, Shin MG, Jang MJ, Cho D, Kee SJ, Kim SH, et al. Comparison between V-Tubes and BD Vacutainer Tubes for Use in Laboratory Tests. Lab Med Online. 2013; 3:145–154.
Article3. CLSI. Validation and Verification of Tubes for Venous and Capillary Blood Specimen Collection; Approved Guideline. CLSI document GP34-A. Wayne, PA: Clinical and Laboratory Standards Institute;2010.4. Ricós C, Alvarez V, Cava F, García-Lario JV, Hernández A, Jiménez CV, et al. Current databases on biological variation: pros, cons and progress. Scand J Clin Lab Invest. 1999; 59:491–500.
Article5. Westgard JO. Desirable Specifications for Total Error, Imprecision, and Bias, derived from intra- and inter-individual biologic variation. Updated on 22 Jun 2014. http://www.westgard.com/biodatabase1.htm.6. CLSI. Method Comparison and Bias Estimation Using Patient Samples Approved Guideline-Second Edition (Interim Revision). CLSI document EP09-A2-IR. Wayne, PA: Clinical and Laboratory Standards Institute;2010.7. CLSI. Tubes and Additives for Venous Blood Specimen Collection; Approved Standard-Fifth Edition. CLSI document H1-A5. Wayne, PA: Clinical and Laboratory Standards Institute;2003.8. Bowen RA, Chan Y, Ruddel ME, Hortin GL, Csako G, Demosky SJ Jr, et al. Immunoassay interference by a commonly used blood collection tube additive, the organosilicone surfactant silwet L-720. Clin Chem. 2005; 51:1874–1882.
Article9. Bowen RA, Hortin GL, Csako G, Otañez OH, Remaley AT. Impact of blood collection devices on clinical chemistry assays. Clin Biochem. 2010; 43:4–25.
Article10. Bowen RA, Vu C, Remaley AT, Hortin GL, Csako G. Differential effect of blood collection tubes on total free fatty acids (FFA) and total triiodothyronine (TT3) concentration: a model for studying interference from tube constituents. Clin Chim Acta. 2007; 378:181–193.
Article11. Bush VJ, Janu MR, Bathur F, Wells A, Dasgupta A. Comparison of BD Vacutainer SST Plus Tubes with BD SST II Plus Tubes for common analytes. Clin Chim Acta. 2001; 306:139–143.
Article12. Li Z, Feng Z, Yan C, Yan R. Evaluation of BD Vacutainer SST™ II Plus tubes for common tumor marker tests by Roche Diagnostics Modular E 170 analyzer. J Clin Lab Anal. 2010; 24:418–421.
Article13. CLSI. Collection, Transport, and Processing of Blood Specimens for Testing Plasma-Based Coagulation Assays and Molecular Hemostasis Assays; Approved Guideline. CLSI Document H21-A5. 5th ed. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute;2008.14. Versalovic J. American Society for Microbiology. Manual of clinical microbiology. 10th ed. Washington, DC: ASM Press;2011. p. 286–301.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Two New Plastic Tubes (Sekisui INSEPACK and Green Cross Green Vac-Tube) with BD Vacutainer Tubes for 49 Analytes
- Comparison of Sekisui Trank Insepack and BD Vacutainer Plastic Citrate Tubes for Routine Coagulation Assays
- Comparison of Improvacuter EDTA Tube with BD Vacutainer EDTA Tube for Routine Hematological Analysis: Clinical Significance of Differences, Stability Study, and Effects of K₂ and K₃ EDTA
- Comparison between V-Tubes and BD Vacutainer Tubes for Use in Laboratory Tests
- Comparison between Medion Vacuon Tube and BD Vacutainer Tube for Clinical Laboratory Practice