Lab Anim Res.
2015 Sep;31(3):111-116. 10.5625/lar.2015.31.3.111.
Effects of pentoxifylline administration on histomorphological parameters of streptozotocin-induced diabetic rat testes
- Affiliations
-
- 1Department of Biology and Anatomical Sciences, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
- 2Cellular and Molecular Biology Research Centre, Shahid Beheshti University of Medical Sciences, Tehran, Iran. mohbayat@sbmu.ac.ir
- KMID: 2312127
- DOI: http://doi.org/10.5625/lar.2015.31.3.111
Abstract
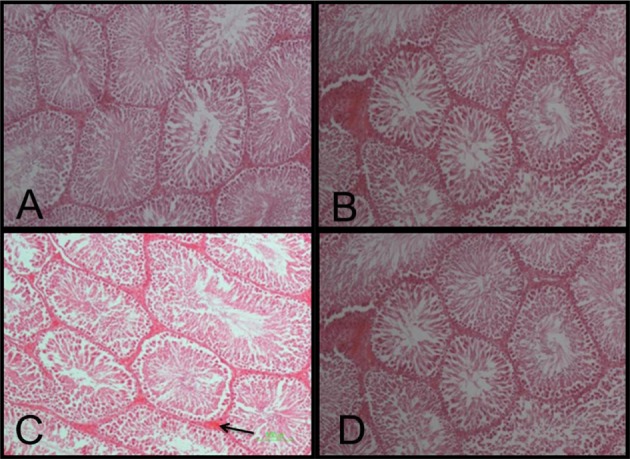
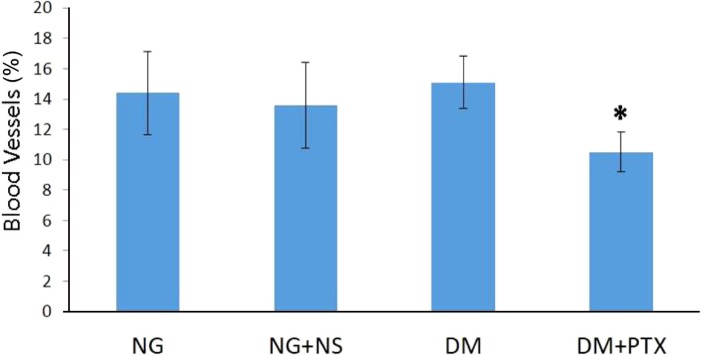
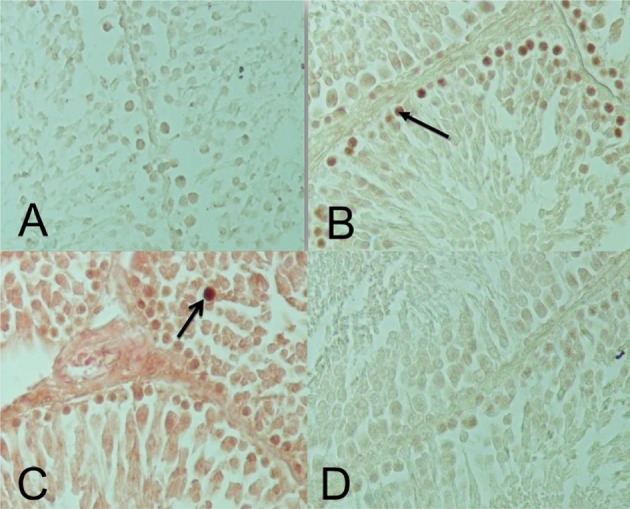
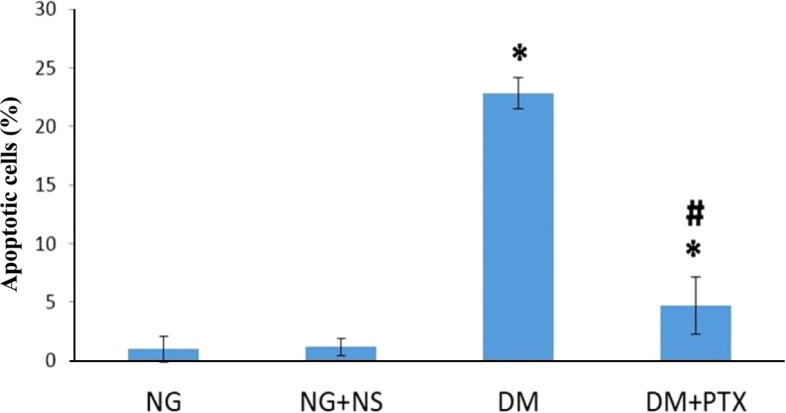
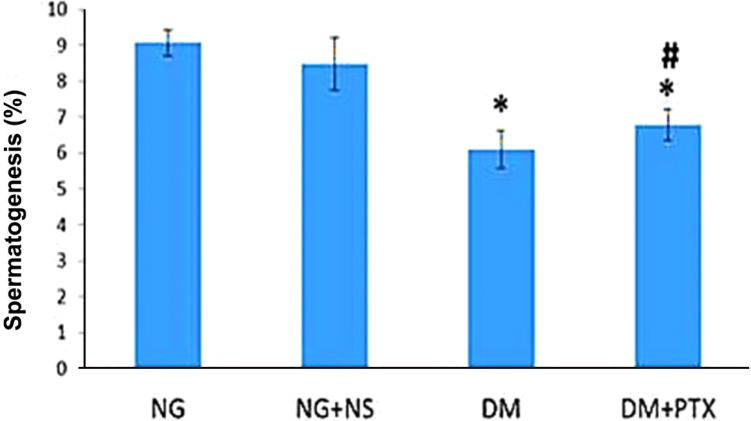
- The effect of pentoxifylline (PTX) administration on histomorphological parameters of streptozotocin (STZ)-induced type 1 diabetes mellitus (DM) in male rat testes were evaluated. We randomly divided 40 male rats into the following four groups: group 1: control or normal glycemic (NG) rats; group 2 or NG rats that received only normal saline (NS), (NG+NS); group 3 or diabetic rats which were not treated by PTX (DM+vehicle solution (NS)); and group 4 which comprised diabetic rats treated with 50 mg/kg of PTX (DM+PTX). Type 1 DM was induced by intraperitoneal injection of STZ (55 mg/kg). Rats were held for 30 days after which the experimental group received PTX twice daily (25 mg/kg) or NS. After 14 days of treatment by PTX or NS, the left testes from all rats were extracted and prpared for histological study. Apoptotic cells, blood vessel density, and spermatogenesis were evaluated. Data were analyzed by ANOVA test. PTX-treated-diabetic rats showed a significant decrease in number of apoptotic cells and decrease in blood vessel density compared to the DM+NS rats. A significant increase in spermatogenesis was observed in the PTX-treated diabetic group, compared to the DM+NS groups. It was concluded that PTX administration to STZ-induced type 1 DM rats affected apoptotic cell number positively. Moreover, blood vessel density significantly decreased and improvements were observed in spermatogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Amaral S, Oliveira PJ, Ramalho-Santos J. Diabetes and the impairment of reproductive function: possible role of mitochondria and reactive oxygen species. Curr Diabetes Rev. 2008; 4(1):46–54. PMID: 18220695.2. Thomay AA, Daley JM, Sabo E, Worth PJ, Shelton LJ, Harty MW, Reichner JS, Albina JE. Disruption of interleukin-1 signaling improves the quality of wound healing. Am J Pathol. 2009; 174(6):2129–2136. PMID: 19389930.
Article3. Dey L, Attele AS, Yuan CS. Alternative therapies for type 2 diabetes. Textbook of complementary and alternative medicine. CRC Press;2003. p. 267.4. Kolodny RC, Kahn CB, Goldstein HH, Barnett DM. Sexual dysfunction in diabetic men. Diabetes. 1974; 23(4):306–309. PMID: 4823910.
Article5. Fairburn C. The sexual problems of diabetic men. Br J Hosp Med. 1981; 25(5):484487489–491. PMID: 7025943.6. Steger RW, Rabe MB. The effect of diabetes mellitus on endocrine and reproductive function. Proc Soc Exp Biol Med. 1997; 214(1):1–11. PMID: 9012356.
Article7. Mosher WD. Fecundity and infertility in the United States. Am J Public Health. 1988; 78(2):181–182. PMID: 3337335.
Article8. Aviado DM, Porter JM. Pentoxifylline: a new drug for the treatment of intermittent claudication. Mechanism of action, pharmacokinetics, clinical efficacy and adverse effects. Pharmacotherapy. 1984; 4(6):297–307. PMID: 6393073.
Article9. Rendell M, Bamisedun O. Skin blood flow and current perception in pentoxifylline-treated diabetic neuropathy. Angiology. 1992; 43(10):843–851. PMID: 1476272.
Article10. Aparicio NJ, Schwarzstein L, de Turner EA. Pentoxifylline (BL 191) by oral administration in the treatment of asthenozoospermia. Andrologia. 1980; 12(3):228–231. PMID: 7004272.
Article11. Babaei S, Bayat M, Nouruzian M, Bayat M. Pentoxifylline improves cutaneous wound healing in streptozotocin-induced diabetic rats. Eur J Pharmacol. 2013; 700(1-3):165–172. PMID: 23220163.
Article12. Soleimani Mehranjani M, Taefi R. The effects of sodium arsenite on the testis structure and sex hormones in vasectomised rats. Iran J Reprod Med. 2012; 10(6):571–580. PMID: 25246929.13. Savaş C, Dindar H, Aras T, Yücesan S. Pentoxifylline improves blood flow to both testes in testicular torsion. Int Urol Nephrol. 2002; 33(1):81–85. PMID: 12090346.14. Najar A, Piryae A, Babaei S, Bayat M. Effect of pentoxifylline on Sertoli and Leydig cells count of experimentally induced type 1 diabetes in male rats. Ann Mil Health Sci Res. 2013; 11(3):188–195.15. Queiroz GCD, Oliveir VVG, Gueiros OG, Torres SM, Maia FCL, Tenorio BM, Morais RN, Silva JVA. Effect of pentoxifylline on the regeneration of rat testicular germ cells after heat shockd. Anim Reprod. 2013; 10(1):45–54.16. Zhao Y, Tan Y, Dai J, Li B, Guo L, Cui J, Wang G, Shi X, Zhang X, Mellen N, Li W, Cai L. Exacerbation of diabetes-induced testicular apoptosis by zinc deficiency is most likely associated with oxidative stress, p38 MAPK activation, and p53 activation in mice. Toxicol Lett. 2011; 200(1-2):100–106. PMID: 21078376.
Article17. Johnsen SG. Testicular biopsy score count--a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones. 1970; 1(1):2–25. PMID: 5527187.18. Xu L, Kanasaki K, Kitada M, Koya D. Diabetic angiopathy and angiogenic defects. Fibrogenesis Tissue Repair. 2012; 5(1):13. PMID: 22853690.
Article19. Pfister F, Feng Y, vom Hagen F, Hoffmann S, Molema G, Hillebrands JL, Shani M, Deutsch U, Hammes HP. Pericyte migration: a novel mechanism of pericyte loss in experimental diabetic retinopathy. Diabetes. 2008; 57(9):2495–2502. PMID: 18559662.20. Guo M, Ricardo SD, Deane JA, Shi M, Cullen-McEwen L, Bertram JF. A stereological study of the renal glomerular vasculature in the db/db mouse model of diabetic nephropathy. J Anat. 2005; 207(6):813–821. PMID: 16367807.
Article21. Nyengaard JR, Rasch R. The impact of experimental diabetes mellitus in rats on glomerular capillary number and sizes. Diabetologia. 1993; 36(3):189–194. PMID: 8462766.
Article22. Nakagawa T, Kosugi T, Haneda M, Rivard CJ, Long DA. Abnormal angiogenesis in diabetic nephropathy. Diabetes. 2009; 58(7):1471–1478. PMID: 19564458.
Article23. Garcia FA, Rebouças JF, Balbino TQ, da Silva TG, de Carvalho-Júnior CH, Cerqueira GS, Brito GA, Viana GS. Pentoxifylline reduces the inflammatory process in diabetic rats: relationship with decreases of pro-inflammatory cytokines and inducible nitric oxide synthase. J Inflamm (Lond). 2015; 12:33. PMID: 25922592.
Article24. Rasslan R, Utiyama EM, Marques GM, Ferreira TC, da Costa VA, de Victo NC, Rasslan S, Montero EF. Inflammatory activity modulation by hypertonic saline and pentoxifylline in a rat model of strangulated closed loop small bowel obstruction. Int J Surg. 2014; 12(6):594–600. PMID: 24797690.
Article25. Ersoy YE, Ayan F, Himmetoglu S. Trace element levels in ischemia-reperfusion injury after left colonic anastomosis in rats and effects of papaverine and pentoxiphylline on vascular endothelial growth factor in anastomosis healing. Acta Gastroenterol Belg. 2011; 74(1):22–27. PMID: 21563650.26. Tahergorabi Z, Khazaei M. Imbalance of angiogenesis in diabetic complications: the mechanisms. Int J Prev Med. 2012; 3(12):827–838. PMID: 23272281.
Article27. Cai L, Chen S, Evans T, Deng DX, Mukherjee K, Chakrabarti S. Apoptotic germ-cell death and testicular damage in experimental diabetes: prevention by endothelin antagonism. Urol Res. 2000; 28(5):342–347. PMID: 11127715.
Article28. Enzlin P, Mathieu C, Van Den Bruel A, Vanderschueren D, Demyttenaere K. Prevalence and predictors of sexual dysfunction in patients with type 1 diabetes. Diabetes Care. 2003; 26(2):409–414. PMID: 12547871.
Article29. Oksanen A. Testicular lesions of streptozotocin diabetic rats. Horm Res. 1975; 6(3):138–144. PMID: 130334.
Article30. Ballester J, Muñoz MC, Domínguez J, Rigau T, Guinovart JJ, Rodríguez-Gil JE. Insulin-dependent diabetes affects testicular function by FSH- and LH-linked mechanisms. J Androl. 2004; 25(5):706–719. PMID: 15292100.
Article31. Ricci G, Catizone A, Esposito R, Pisanti FA, Vietri MT, Galdieri M. Diabetic rat testes: morphological and functional alterations. Andrologia. 2009; 41(6):361–368. PMID: 19891634.
Article32. Bal R, Türk G, Tuzcu M, Yilmaz O, Ozercan I, Kuloglu T, Gür S, Nedzvetsky VS, Tykhomyrov AA, Andrievsky GV, Baydas G, Naziroglu M. rotective effects of nanostructures of hydrated C(60) fullerene on reproductive function in streptozotocindiabetic male rats. Toxicology. 2011; 282(3):69–81. PMID: 21163323.33. McKinney KA, Lewis SE, Thompson W. Persistent effects of pentoxifylline on human sperm motility, after drug removal, in normozoospermic and asthenozoospermic individuals. Andrologia. 1994; 26(4):235–240. PMID: 7978376.
Article34. Terriou P, Hans E, Giorgetti C, Spach JL, Salzmann J, Urrutia V, Roulier R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J Assist Reprod Genet. 2000; 17(4):194–199. PMID: 10955242.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Protective Effect of Melatonin on Neuropathy in Streptozotocin-Induced Diabetic Rats
- Proliferation of Cultured Vascular Smooth Muscle Cells(VSMCs) Obtained from Aortas of Insulin Dependent Diabetic Rats
- The effects of pentoxifylline of the flap tolerance to arterial and venous ischemia in the diabetic rats
- The renoprotective effects of pentoxifylline: beyond its role in diabetic nephropathy
- The Effects of Insulin Treatment on the Contractile Responses of the Seminal Vesicle in Streptozotocin-induced Diabetic Rats