J Dent Anesth Pain Med.
2015 Dec;15(4):221-227.
Optimal effect-site concentration of remifentanil for minimizing cardiovascular changes caused by fiberoptic nasotracheal intubation
- Affiliations
-
- 1Department of Dental Anesthesia and Pain Medicine, Pusan National University Dental Hospital, Dental Research Institute, Yangsan, Korea. dryoonji@pusan.ac.kr
- 2Department of Anesthesia and Pain Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea.
Abstract
- BACKGROUND
Endotracheal intubation induces clinically adverse cardiovascular changes. Various pharmacological strategies for controlling these responses have been suggested with opioids being widely administered. In this study, the optimal effect-site concentration (Ce) of remifentanil for minimizing hemodynamic responses to fiberoptic nasotracheal intubation was evaluated.
METHODS
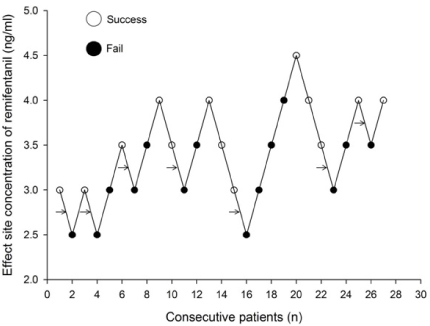
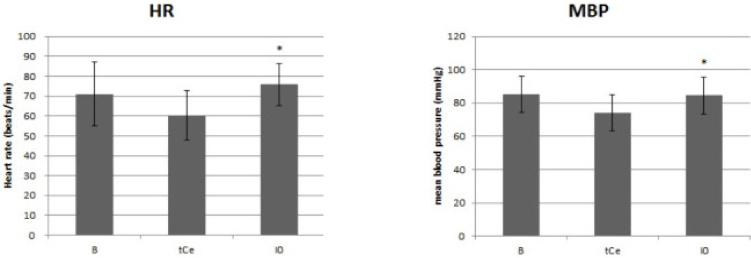
Thirty patients, aged 18-63 years, scheduled for elective surgery were included. Anesthesia was induced with a propofol and remifentanil infusion via target-controlled infusion (TCI). Remifentanil infusion was initiated at 3.0 ng/mL, and the response of each patient determined the Ce of remifentanil for the next patient by the Dixon up-and-down method at an interval of 0.5 ng/mL. Rocuronium was administered after propofol and remifentanil reached their preset Ce; 90 seconds later fiberoptic nasotracheal intubation was initiated. Non-invasive blood pressure and heart rate (HR) were measured at pre-induction, the time Ce was reached, immediately before and after intubation, and at 1 and 3 minutes after intubation. The up-and-down criteria comprised a 20% change in mean blood pressure and HR between just prior to intubation and 1 minute after intubation.
RESULTS
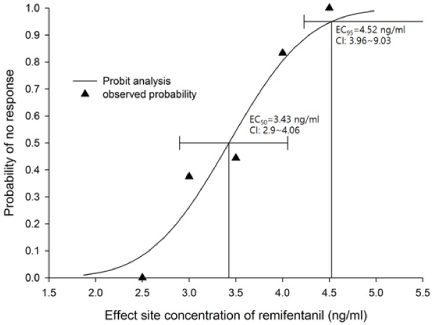
The median effective effect-site concentration (EC50) of remifentanil was 3.11 ± 0.38 ng/mL by the Dixon's up-and-down method. From the probit analysis, the EC50 of remifentanil was 3.43 ng/mL (95% confidence interval, 2.90-4.06 ng/mL). In PAVA, the EC50 and EC95 of remifentanil were 3.57 ng/mL (95% CI, 2.95-3.89) and 4.35 ng/mL (95% CI, 3.93-4.45). No remifentanil-related complications were observed.
CONCLUSIONS
The EC50 of remifentanil for minimizing the cardiovascular changes and side effects associated with fiberoptic nasotracheal intubation was 3.11-3.43 ng/mL during propofol TCI anesthesia with a Ce of 4 ug/mL.
Keyword
MeSH Terms
Figure
Reference
-
1. Hall CE, Shutt LE. Nasotracheal intubation for head and neck surgery. Anaesthesia. 2003; 58:249–256.
Article2. Matsuura H, Hirose I, Joh S, Sugiyama K, Niwa H, Shibutani T. A report of 14,195 applications of anesthetics to oral and maxillofacial surgery at one teaching dental hospital (1971–2000) centering around airway problems. J Clin Anesth. 2000; 12:460–467.
Article3. Tuzuner-Oncul AM, Kucukyavuz Z. Prevalence and prediction of difficult intubation in maxillofacial surgery patients. J Oral Maxillofac Surg. 2008; 66:1652–1658.
Article4. Dong Y, Li G, Wu W, Su R, Shao Y. Lightwand-guided nasotracheal intubation in oromaxillofacial surgery patients with anticipated difficult airways: A comparison with blind nasal intubation. Int J Oral Maxillofac Surg. 2013; 42:1049–1053.
Article5. Umesh G, Manjunath P, Kini G, Jasvinder K. A technique to overcome inability to advance a tracheal tube over a fiberscope during nasotracheal intubation. J Anesth. 2010; 24:819–820.
Article6. Shibata Y, Okamoto K, Matsumoto M, Suzuki K, Sadanaga M, Morioka T. Cardiovascular responses to fiberoptic intubation: A comparison of orotracheal and nasotracheal intubation. J Anesth. 1992; 6:262–268.
Article7. Roy WL, Edelist G, Gilbert B. Myocardial ischemia during non-cardiac surgical procedures in patients with coronaryartery disease. Anesthesiology. 1979; 51:393–397.
Article8. Stoelting RK. Blood pressure and heart rate changes during short-duration laryngoscopy for tracheal intubation: Influence of viscous or intravenous lidocaine. Anesth Analg. 1978; 57:197–199.9. Siedlecki J. Disturbances in the function of cardiovascular system in patients following endotracheal intubation and attempts of their prevention by pharmacological bloackade of sympathetic system. Anaesth Resusc Intensive Ther. 1975; 3:107–123.10. Stanley TH, Berman L, Green O, Robertson D. Plasma catecholamine and cortisol responses to fentanyl--oxygen anesthesia for coronary-artery operations. Anesthesiology. 1980; 53:250–253.
Article11. Hayashi Y, Maze M. Alpha 2 adrenoceptor agonists and anaesthesia. Br J Anaesth. 1993; 71:108–118.
Article12. Ghignone M, Quintin L, Duke PC, Kehler CH, Calvillo O. Effects of clonidine on narcotic requirements and hemodynamic response during induction of fentanyl anesthesia and endotracheal intubation. Anesthesiology. 1986; 64:36–42.
Article13. Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988; 150:9–14.
Article14. Jaakola ML, Ali-Melkkila T, Kanto J, Kallio A, Scheinin H, Scheinin M. Dexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgery. Br J Anaesth. 1992; 68:570–575.
Article15. Lawrence CJ, De Lange S. Effects of a single pre-operative dexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability. Anaesthesia. 1997; 52:736–744.
Article16. Beers R, Camporesi E. Remifentanil update: Clinical science and utility. CNS Drugs. 2004; 18:1085–1104.17. Glass PS, Hardman D, Kamiyama Y, Quill TJ, Marton G, Donn KH, et al. Preliminary pharmacokinetics and pharmacodynamics of an ultra-short-acting opioid: Remifentanil (GI87084B). Anesth Analg. 1993; 77:1031–1040.18. Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980; 20:441–462.
Article19. Dixon WJ. Staircase bioassay: The up-and-down method. Neurosci Biobehav Rev. 1991; 15:47–50.
Article20. Lee J, Jung C. The target concentration of remifentanil to suppress the hemodynamic response to endotracheal intubation during inhalational induction with desflurane. Korean J Anesthesiol. 2011; 60:12–18.
Article21. Shribman AJ, Smith G, Achola KJ. Cardiovascular and catecholamine responses to laryngoscopy with and without tracheal intubation. Br J Anaesth. 1987; 59:295–299.
Article22. Edwards ND, Alford AM, Dobson PM, Peacock JE, Reilly CS. Myocardial ischaemia during tracheal intubation and extubation. Br J Anaesth. 1994; 73:537–539.
Article23. Mikawa K, Nishina K, Maekawa N, Obara H. Comparison of nicardipine, diltiazem and verapamil for controlling the cardiovascular responses to tracheal intubation. Br J Anaesth. 1996; 76:221–226.
Article24. Yoo KY, Lee JU, Kim HS, Im WM. Hemodynamic and catecholamine responses to laryngoscopy and tracheal intubation in patients with complete spinal cord injuries. Anesthesiology. 2001; 95:647–651.
Article25. Burney RG, Winn R. Increased cerbrospinal fluid pressure during laryngoscopy and intubation for induction of anesthesia. Anesth Analg. 1975; 54:687–690.26. Singh S, Smith JE. Cardiovascular changes after the three stages of nasotracheal intubation. Br J Anaesth. 2003; 91:667–671.
Article27. Roy WL, Edelist G, Gilbert B. Myocardial ischemia during non-cardiac surgical procedures in patients with coronary-artery disease. Anesthesiology. 1979; 51:393–397.
Article28. Fassoulaki A, Andreopoulou K, Saleh M, Kitharitzi D. Metabolic and cardiovascular responses following oral and nasal intubation of the trachea. Acta Anaesthesiol Belg. 1990; 41:281–286.29. Burkle H, Dunbar S, Van Aken H. Remifentanil: A novel, short-acting, mu-opioid. Anesth Analg. 1996; 83:646–651.30. Lallo A, Billard V, Bourgain JL. A comparison of propofol and remifentanil target-controlled infusions to facilitate fiberoptic nasotracheal intubation. Anesth Analg. 2009; 108:852–857.
Article31. Kim KO, Chung S, Chang EJ, Lee Y. Optimal effect-site concentration of remifentanil for preventing development of hypertension during tracheal intubation with inhaled desflurane induction. Korean J Anesthesiol. 2011; 60:8–11.
Article32. Smith C, McEwan AI, Jhaveri R, Wilkinson M, Goodman D, Smith LR, et al. The interaction of fentanyl on the Cp50 of propofol for loss of consciousness and skin incision. Anesthesiology. 1994; 81:820–828.
Article33. Vuyk J, Engbers FH, Burm AG, Vletter AA, Griever GE, Olofsen E, et al. Pharmacodynamic interaction between propofol and alfentanil when given for induction of anesthesia. Anesthesiology. 1996; 84:288–299.
Article34. Kil HY, Lee SI, Lee SJ, Lee SW, Lee DH. The bispectral index and modified observer's assessment of alertness/sedation scale comparable to effect site concentration of propofol in koreans. Korean J Anesthesiol. 2000; 38:251–257.
Article35. Stylianou M. Sequential analysis of durham and Flournoy's biased coin design for phase I clinical trials. American University;2000. p. 356.36. Paul M, Fisher DM. Are estimates of MAC reliable? Anesthesiology. 2001; 95:1362–1370.
Article37. Stylianou M, Flournoy N. Dose finding using the biased coin Up–and–Down design and isotonic regression. Biometrics. 2002; 58:171–177.
Article38. Robertson T, Wright F, Dykstra RL. Order restricted statistical inference. Wiley New York. 1988; 30:316.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal effect-site concentration of remifentanil to prevent hemodynamic changes during nasotracheal intubation using a video laryngoscope
- What is the optimal effect-site concentration of remifentanil for minimizing the cardiovascular changes to endotracheal intubation during induction with propofol in elderly patients?
- Effective removal of epistaxis during nasotracheal intubation utilizing a fiberoptic scope in a difficult airway: A case report
- Blind Intubation Using Fiberoptic Bronchoscope in Epistaxis
- What is the Optimal Effect-site Concentration of Remifentanil for Minimizing the Cardiovascular Changes to Endotracheal Intubation during Induction with Propofol?