Korean J Physiol Pharmacol.
2014 Feb;18(1):73-78. 10.4196/kjpp.2014.18.1.73.
Bcl-2 Knockdown Accelerates T Cell Receptor-Triggered Activation-Induced Cell Death in Jurkat T Cells
- Affiliations
-
- 1Laboratory of Host Defense Modulation, College of Pharmacy, Chung-Ang University, Seoul 156-756, Korea. khwang@cau.ac.kr
- KMID: 2285494
- DOI: http://doi.org/10.4196/kjpp.2014.18.1.73
Abstract
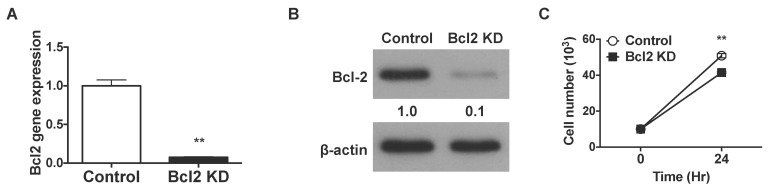
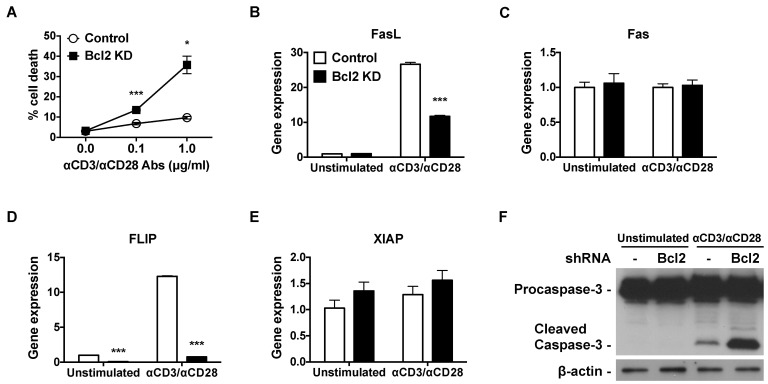
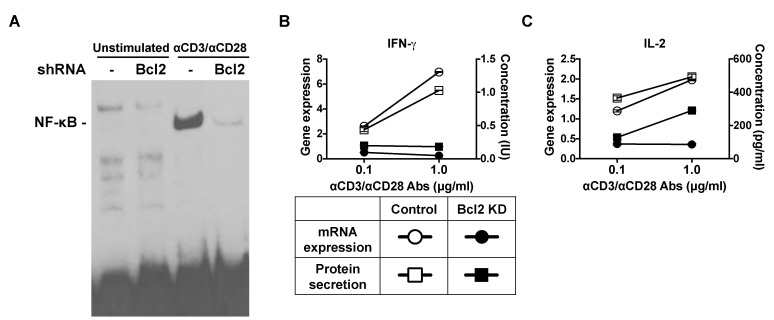
- Cell death and survival are tightly controlled through the highly coordinated activation/inhibition of diverse signal transduction pathways to insure normal development and physiology. Imbalance between cell death and survival often leads to autoimmune diseases and cancer. Death receptors sense extracellular signals to induce caspase-mediated apoptosis. Acting upstream of CED-3 family proteases, such as caspase-3, Bcl-2 prevents apoptosis. Using short hairpin RNAs (shRNAs), we suppressed Bcl-2 expression in Jurkat T cells, and this increased TCR-triggered AICD and enhanced TNFR gene expression. Also, knockdown of Bcl-2 in Jurkat T cells suppressed the gene expression of FLIP, TNF receptor-associated factors 3 (TRAF3) and TRAF4. Furthermore, suppressed Bcl-2 expression increased caspase-3 and diminished nuclear factor kappa B (NF-kappaB) translocation.
MeSH Terms
-
Apoptosis
Autoimmune Diseases
Caspase 3
Cell Death*
Gene Expression
Humans
NF-kappa B
Peptide Hydrolases
Physiology
Receptors, Death Domain
RNA, Small Interfering
Signal Transduction
T-Lymphocytes*
TNF Receptor-Associated Factor 4
Tumor Necrosis Factor Receptor-Associated Peptides and Proteins
Caspase 3
NF-kappa B
Peptide Hydrolases
RNA, Small Interfering
Receptors, Death Domain
TNF Receptor-Associated Factor 4
Tumor Necrosis Factor Receptor-Associated Peptides and Proteins
Figure
Reference
-
1. Van Parijs L, Biuckians A, Abbas AK. Functional roles of Fas and Bcl-2-regulated apoptosis of T lymphocytes. J Immunol. 1998; 160:2065–2071. PMID: 9498742.2. Rieux-Laucat F, Le Deist F, Fischer A. Autoimmune lymphoproliferative syndromes: genetic defects of apoptosis pathways. Cell Death Differ. 2003; 10:124–133. PMID: 12655301.
Article3. Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002; 2:277–288. PMID: 12001989.
Article4. Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998; 281:1305–1308. PMID: 9721089.
Article5. Schmitz I, Kirchhoff S, Krammer PH. Regulation of death receptor-mediated apoptosis pathways. Int J Biochem Cell Biol. 2000; 32:1123–1136. PMID: 11137452.
Article6. Itoh N, Yonehara S, Ishii A, Yonehara M, Mizushima S, Sameshima M, Hase A, Seto Y, Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991; 66:233–243. PMID: 1713127.
Article7. Trauth BC, Klas C, Peters AM, Matzku S, Möller P, Falk W, Debatin KM, Krammer PH. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989; 245:301–305. PMID: 2787530.
Article9. Russell JH, Wang R. Autoimmune gld mutation uncouples suicide and cytokine/proliferation pathways in activated, mature T cells. Eur J Immunol. 1993; 23:2379–2382. PMID: 8370416.
Article10. Russell JH, Rush B, Weaver C, Wang R. Mature T cells of autoimmune lpr/lpr mice have a defect in antigen-stimulated suicide. Proc Natl Acad Sci U S A. 1993; 90:4409–4413. PMID: 8506280.
Article11. Bossu P, Singer GG, Andres P, Ettinger R, Marshak-Rothstein A, Abbas AK. Mature CD4+ T lymphocytes from MRL/lpr mice are resistant to receptor-mediated tolerance and apoptosis. J Immunol. 1993; 151:7233–7239. PMID: 7903104.12. Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995; 181:71–77. PMID: 7528780.
Article13. Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001; 11:372–377. PMID: 11514191.
Article14. Beutler B, Cerami A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annu Rev Biochem. 1988; 57:505–518. PMID: 3052281.
Article15. Beutler B, Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989; 7:625–655. PMID: 2540776.17. Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992; 10:411–452. PMID: 1590993.
Article18. Laster SM, Wood JG, Gooding LR. Tumor necrosis factor can induce both apoptic and necrotic forms of cell lysis. J Immunol. 1988; 141:2629–2634. PMID: 3171180.19. Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thornberry NA, Wong WW, Yuan J. Human ICE/CED-3 protease nomenclature. Cell. 1996; 87:171. PMID: 8861900.
Article20. Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997; 275:1129–1132. PMID: 9027314.
Article21. Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG, Dixit VM. Molecular ordering of the cell death pathway. Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996; 271:4573–4576. PMID: 8617712.22. Erhardt P, Cooper GM. Activation of the CPP32 apoptotic protease by distinct signaling pathways with differential sensitivity to Bcl-xL. J Biol Chem. 1996; 271:17601–17604. PMID: 8663611.
Article23. Armstrong RC, Aja T, Xiang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewsky DS, Fritz LC, Tomaselli KJ. Fas-induced activation of the cell death-related protease CPP32 Is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996; 271:16850–16855. PMID: 8663439.
Article24. Shimizu S, Eguchi Y, Kamiike W, Matsuda H, Tsujimoto Y. Bcl-2 expression prevents activation of the ICE protease cascade. Oncogene. 1996; 12:2251–2257. PMID: 8649764.25. Vaux DL, Weissman IL, Kim SK. Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science. 1992; 258:1955–1957. PMID: 1470921.26. Gaumer S, Guénal I, Brun S, Théodore L, Mignotte B. Bcl-2 and Bax mammalian regulators of apoptosis are functional in Drosophila. Cell Death Differ. 2000; 7:804–814. PMID: 11042675.
Article27. Hengartner MO, Ellis RE, Horvitz HR. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992; 356:494–499. PMID: 1560823.
Article28. Hengartner MO, Horvitz HR. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994; 76:665–676. PMID: 7907274.
Article29. Langenau DM, Jette C, Berghmans S, Palomero T, Kanki JP, Kutok JL, Look AT. Suppression of apoptosis by bcl-2 overexpression in lymphoid cells of transgenic zebrafish. Blood. 2005; 105:3278–3285. PMID: 15618471.
Article30. Nicolas O, Gavín R, Braun N, Ureña JM, Fontana X, Soriano E, Aguzzi A, del Río JA. Bcl-2 overexpression delays caspase-3 activation and rescues cerebellar degeneration in prion-deficient mice that overexpress amino-terminally truncated prion. FASEB J. 2007; 21:3107–3117. PMID: 17494993.
Article31. Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999; 6:99–104. PMID: 10200555.
Article32. Li Q, Ching AK, Chan BC, Chow SK, Lim PL, Ho TC, Ip WK, Wong CK, Lam CW, Lee KK, Chan JY, Chui YL. A death receptor-associated anti-apoptotic protein, BRE, inhibits mitochondrial apoptotic pathway. J Biol Chem. 2004; 279:52106–52116. PMID: 15465831.
Article33. Cui X, Hawari F, Alsaaty S, Lawrence M, Combs CA, Geng W, Rouhani FN, Miskinis D, Levine SJ. Identification of ARTS-1 as a novel TNFR1-binding protein that promotes TNFR1 ectodomain shedding. J Clin Invest. 2002; 110:515–526. PMID: 12189246.
Article34. Xu Y, Cheng G, Baltimore D. Targeted disruption of TRAF3 leads to postnatal lethality and defective T-dependent immune responses. Immunity. 1996; 5:407–415. PMID: 8934568.
Article35. Liao G, Zhang M, Harhaj EW, Sun SC. Regulation of the NF-kappaB-inducing kinase by tumor necrosis factor receptor-associated factor 3-induced degradation. J Biol Chem. 2004; 279:26243–26250. PMID: 15084608.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tacrolimus (FK506) Induced Apoptotic Signal Transduction Pathway
- Mycophenolic Acid Induced Apoptosis in Human Jurkat Cells viathe Generation of Reactive Oxygen Species
- Cell Beath Induced by Ethanol : Prevention of Cell Death by the bcl-2 Proto-Oncogene
- Charged MVB protein 5 is involved in T-cell receptor signaling
- Naegleria fowleri Induces Jurkat T Cell Death via O-deGlcNAcylation