Korean J Physiol Pharmacol.
2013 Aug;17(4):299-306. 10.4196/kjpp.2013.17.4.299.
Neuronal Responses in the Globus Pallidus during Subthalamic Nucleus Electrical Stimulation in Normal and Parkinson's Disease Model Rats
- Affiliations
-
- 1Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju 220-710, Korea. khkim0604@yonsei.ac.kr
- 2Brain Korea 21 Projet for Medical Science and Brain Research Institute, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 3Department of Neurosurgery, Yonsei University College of Medicine, Seoul 120-752, Korea.
- 4Department of Physiology, College of Medicine, Hallym University, Chuncheon 200-702, Korea.
- KMID: 2285464
- DOI: http://doi.org/10.4196/kjpp.2013.17.4.299
Abstract
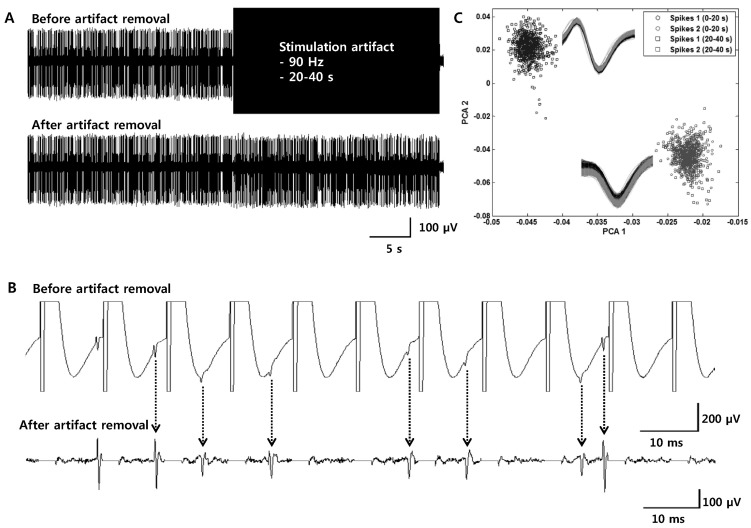
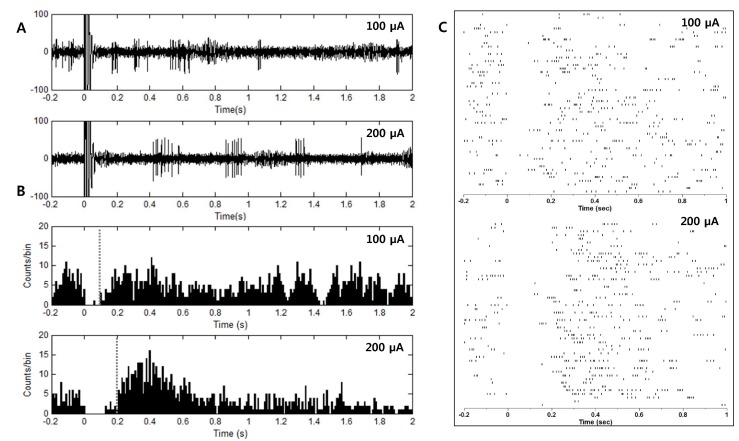
- Deep brain stimulation (DBS) of the subthalamic nucleus (STN) has been widely used as a treatment for the movement disturbances caused by Parkinson's disease (PD). Despite successful application of DBS, its mechanism of therapeutic effect is not clearly understood. Because PD results from the degeneration of dopamine neurons that affect the basal ganglia (BG) network, investigation of neuronal responses of BG neurons during STN DBS can provide informative insights for the understanding of the mechanism of therapeutic effect. However, it is difficult to observe neuronal activity during DBS because of large stimulation artifacts. Here, we report the observation of neuronal activities of the globus pallidus (GP) in normal and PD model rats during electrical stimulation of the STN. A custom artifact removal technique was devised to enable monitoring of neural activity during stimulation. We investigated how GP neurons responded to STN stimulation at various stimulation frequencies (10, 50, 90 and 130 Hz). It was observed that activities of GP neurons were modulated by stimulation frequency of the STN and significantly inhibited by high frequency stimulation above 50 Hz. These findings suggest that GP neuronal activity is effectively modulated by STN stimulation and strongly dependent on the frequency of stimulation.
Keyword
MeSH Terms
Figure
Reference
-
1. The National Collaborating Centre for Chronic Conditions, National Clinical Guideline for Diagnosis and Management in Primary and Secondary Care. London: Royal College of Physicians (UK);2006.2. Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de Rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991; 337:403–406. PMID: 1671433.
Article3. Koller W, Pahwa R, Busenbark K, Hubble J, Wilkinson S, Lang A, Tuite P, Sime E, Lazano A, Hauser R, Malapira T, Smith D, Tarsy D, Miyawaki E, Norregaard T, Kormos T, Olanow CW. High-frequency unilateral thalamic stimulation in the treatment of essential and parkinsonian tremor. Ann Neurol. 1997; 42:292–299. PMID: 9307249.
Article4. Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999; 53:561–566. PMID: 10449121.
Article5. Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect of parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995; 345:91–95. PMID: 7815888.6. Rodriguez-Oroz MC, Obeso JA, Lang AE, Houeto JL, Pollak P, Rehncrona S, Kulisevsky J, Albanese A, Volkmann J, Hariz MI, Quinn NP, Speelman JD, Guridi J, Zamarbide I, Gironell A, Molet J, Pascual-Sedano B, Pidoux B, Bonnet AM, Agid Y, Xie J, Benabid AL, Lozano AM, Saint-Cyr J, Romito L, Contarino MF, Scerrati M, Fraix V, Van Blercom N. Bilateral deep brain stimulation in Parkinson's disease: a multicentre study with 4 years follow-up. Brain. 2005; 128:2240–2249. PMID: 15975946.
Article7. Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994; 35:1126–1129. PMID: 7885558.8. Yianni J, Bain P, Giladi N, Auca M, Gregory R, Joint C, Nandi D, Stein J, Scott R, Aziz T. Globus pallidus internus deep brain stimulation for dystonic conditions: a prospective audit. Mov Disord. 2003; 18:436–442. PMID: 12671953.
Article9. Benazzouz A, Gao DM, Ni ZG, Piallat B, Bouali-Benazzouz R, Benabid AL. Effect of high-frequency stimulation of the subthalamic nucleus on the neuronal activities of the substantia nigra pars reticulata and ventrolateral nucleus of the thalamus in the rat. Neuroscience. 2000; 99:289–295. PMID: 10938434.
Article10. Benazzouz A, Piallat B, Pollak P, Benabid AL. Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci Lett. 1995; 189:77–80. PMID: 7609923.
Article11. Dostrovsky JO, Lozano AM. Mechanisms of deep brain stimulation. Mov Disord. 2002; 17(Suppl 3):S63–S68. PMID: 11948756.
Article12. Maurice N, Thierry AM, Glowinski J, Deniau JM. Spontaneous and evoked activity of substantia nigra pars reticulata neurons during high-frequency stimulation of the subthalamic nucleus. J Neurosci. 2003; 23:9929–9936. PMID: 14586023.
Article13. Tai CH, Boraud T, Bezard E, Bioulac B, Gross C, Benazzouz A. Electrophysiological and metabolic evidence that high-frequency stimulation of the subthalamic nucleus bridles neuronal activity in the subthalamic nucleus and the substantia nigra reticulata. FASEB J. 2003; 17:1820–1830. PMID: 14519661.
Article14. Benabid AL, Pollak P, Gao D, Hoffmann D, Limousin P, Gay E, Payen I, Benazzouz A. Chronic electrical stimulation of the ventralis intermedius nucleus of the thalamus as a treatment of movement disorders. J Neurosurg. 1996; 84:203–214. PMID: 8592222.
Article15. Benabid AL, Pollak P, Gross C, Hoffmann D, Benazzouz A, Gao DM, Laurent A, Gentil M, Perret J. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson's disease. Stereotact Funct Neurosurg. 1994; 62:76–84. PMID: 7631092.
Article16. Vitek JL, Hashimoto T, Peoples J, DeLong MR, Bakay RA. Acute stimulation in the external segment of the globus pallidus improves parkinsonian motor signs. Mov Disord. 2004; 19:907–915. PMID: 15300655.
Article17. Windels F, Bruet N, Poupard A, Urbain N, Chouvet G, Feuerstein C, Savasta M. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. Eur J Neurosci. 2000; 12:4141–4146. PMID: 11069610.
Article18. Shi LH, Luo F, Woodward DJ, Chang JY. Basal ganglia neural responses during behaviorally effective deep brain stimulation of the subthalamic nucleus in rats performing a treadmill locomotion test. Synapse. 2006; 59:445–457. PMID: 16521122.
Article19. Harding GW. A method for eliminating the stimulus artifact from digital recordings of the direct cortical response. Comput Biomed Res. 1991; 24:183–195. PMID: 2036783.
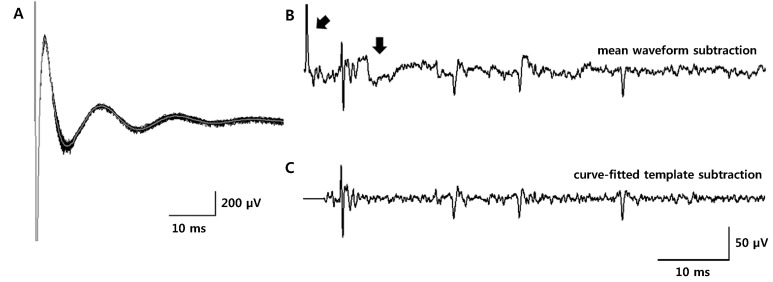
Article20. Hashimoto T, Elder CM, Vitek JL. A template subtraction method for stimulus artifact removal in high-frequency deep brain stimulation. J Neurosci Methods. 2002; 113:181–186. PMID: 11772439.
Article21. Heffer LF, Fallon JB. A novel stimulus artifact removal technique for high-rate electrical stimulation. J Neurosci Methods. 2008; 170:277–284. PMID: 18339428.
Article22. O'Keeffe DT, Lyons GM, Donnelly AE, Byrne CA. Stimulus artifact removal using a software-based two-stage peak detection algorithm. J Neurosci Methods. 2001; 109:137–145. PMID: 11513948.23. Wichmann T. A digital averaging method for removal of stimulus artifacts in neurophysiologic experiments. J Neurosci Methods. 2000; 98:57–62. PMID: 10837871.
Article24. Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson's disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999; 88:617–628. PMID: 10197780.
Article25. DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990; 13:281–285. PMID: 1695404.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Alterations of Spontaneous Behaviors and the Neuronal Activities of the Deep Cerebral Nuclei by Subthalamic Lesion with Kainic Acid in Rat Parkinsonian Models with 6-hydroxydopamine
- Surgical Treatment of Advanced Parkinson Disease
- Comparison of Pallidal and Subthalamic Deep Brain Stimulation in Parkinson's Disease: Therapeutic and Adverse Effects
- Deep Brain Stimulation of the Subthalamic and Pedunculopontine Nucleus in a Patient with Parkinson's Disease
- A Case of Hemiballism