Korean J Physiol Pharmacol.
2010 Jun;14(3):177-183. 10.4196/kjpp.2010.14.3.177.
Tetrahydrobiopterin Inhibits PDGF-stimulated Migration and Proliferation in Rat Aortic Smooth Muscle Cells via the Nitric Oxide Synthase-independent Pathway
- Affiliations
-
- 1Department of Physiology and Biotechnology, Konkuk University, Chungju 380-701, Korea. kjwon@kku.ac.kr
- 2Department of Cosmetic Science, College of Natural Science, Hoseo University, Asan 336-795, Korea. kacsital@hoseo.edu
- KMID: 2285400
- DOI: http://doi.org/10.4196/kjpp.2010.14.3.177
Abstract
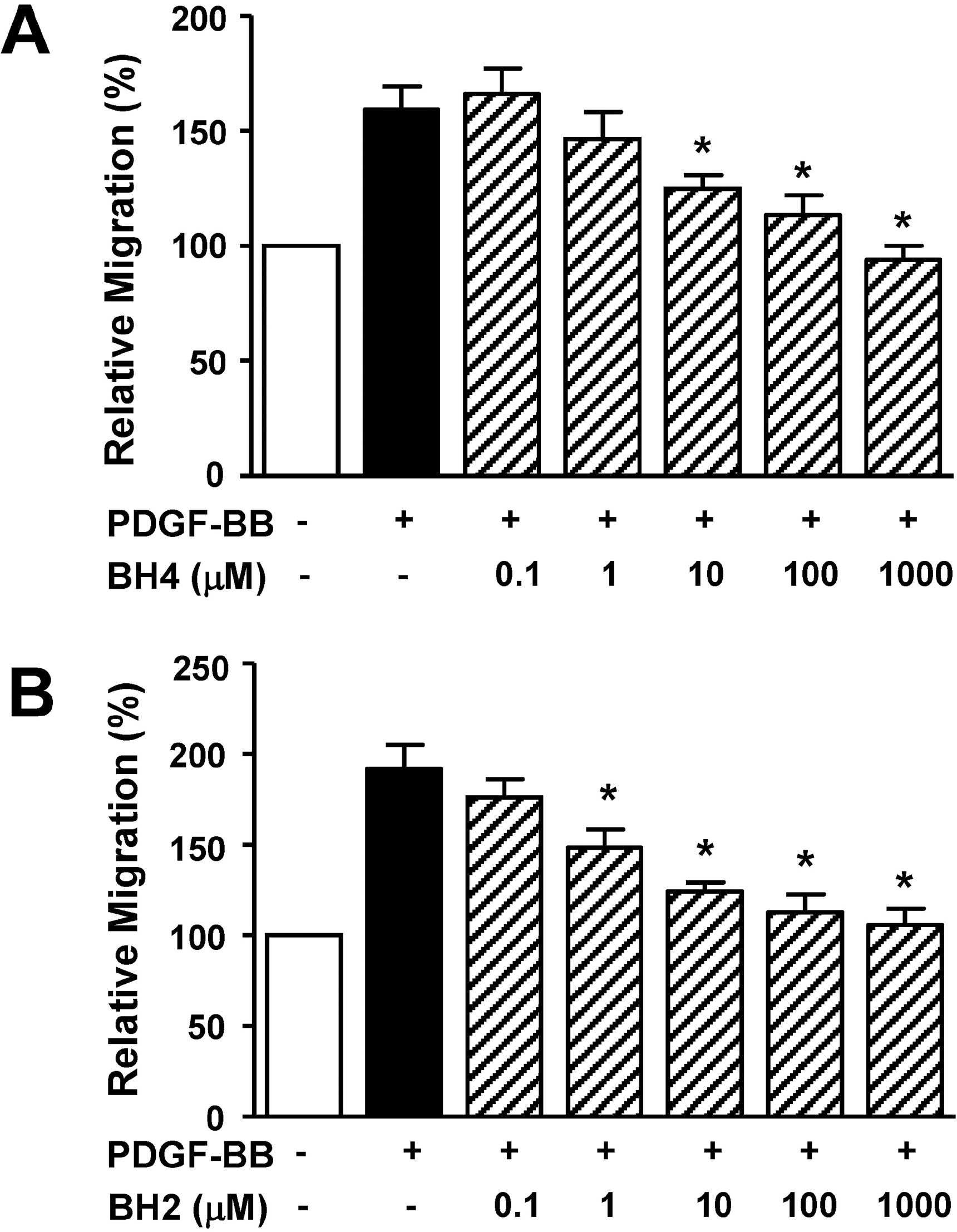
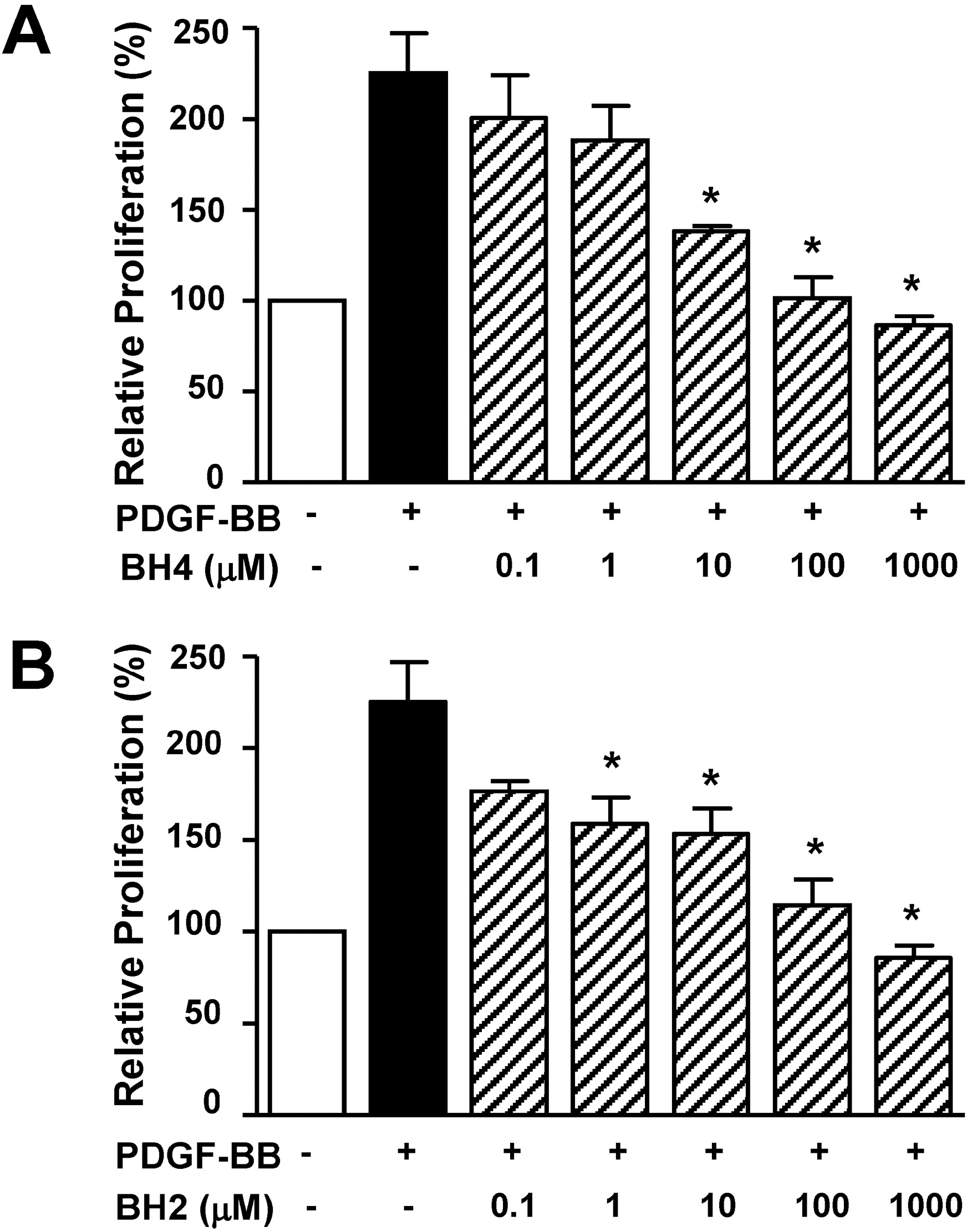
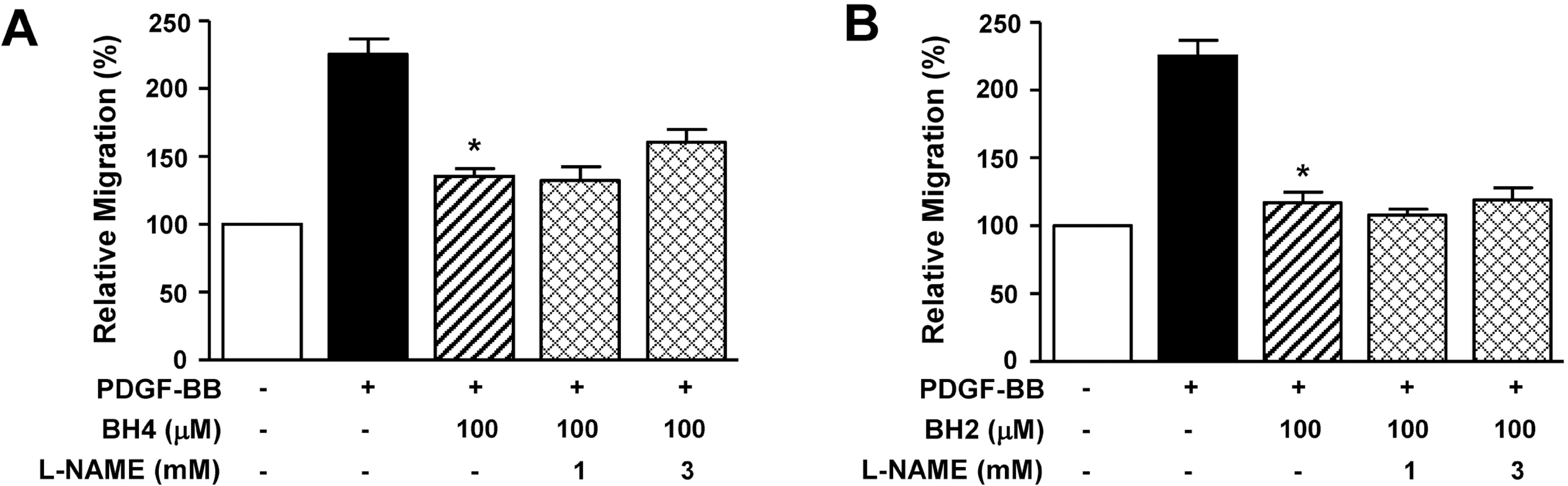
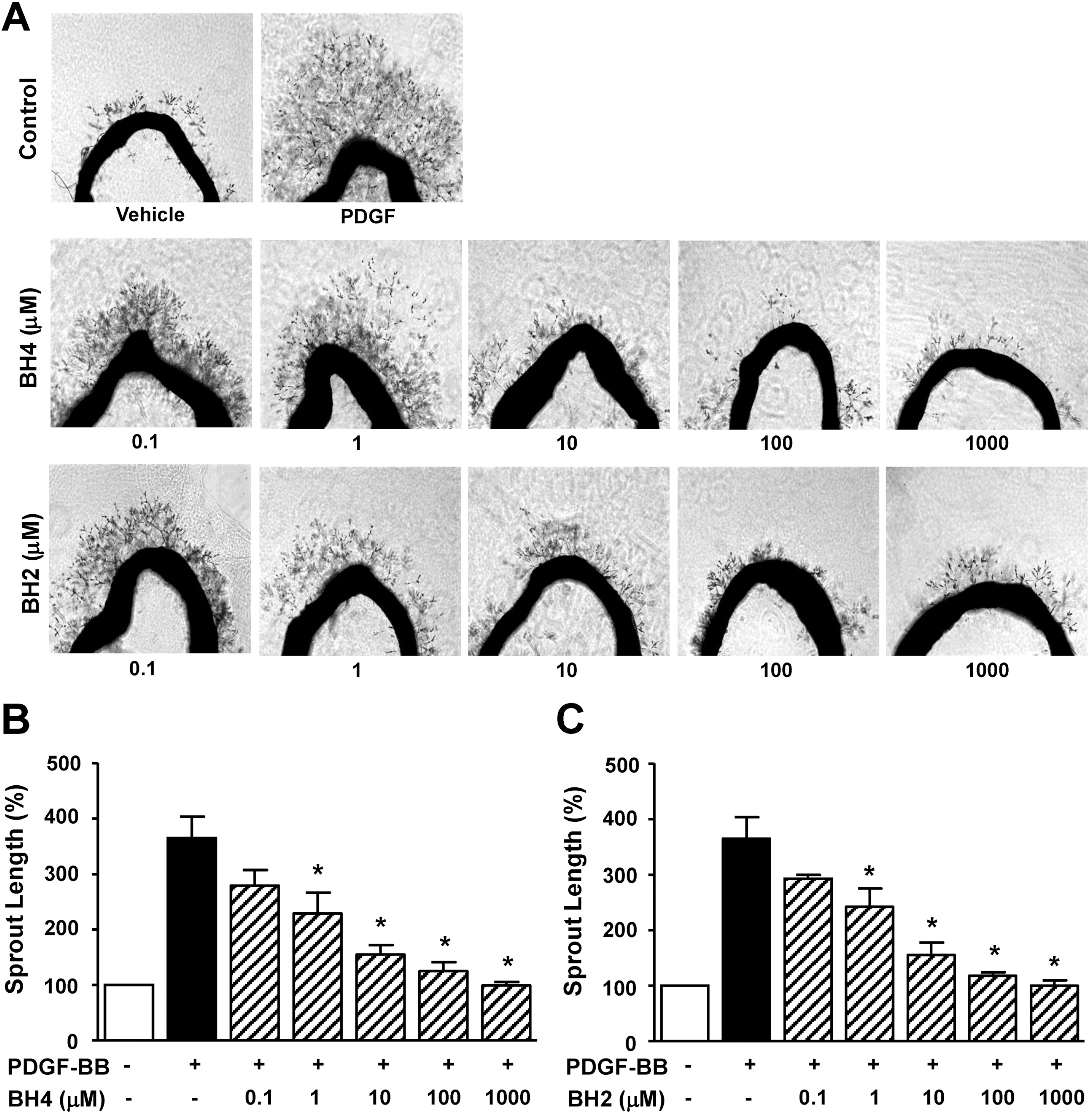
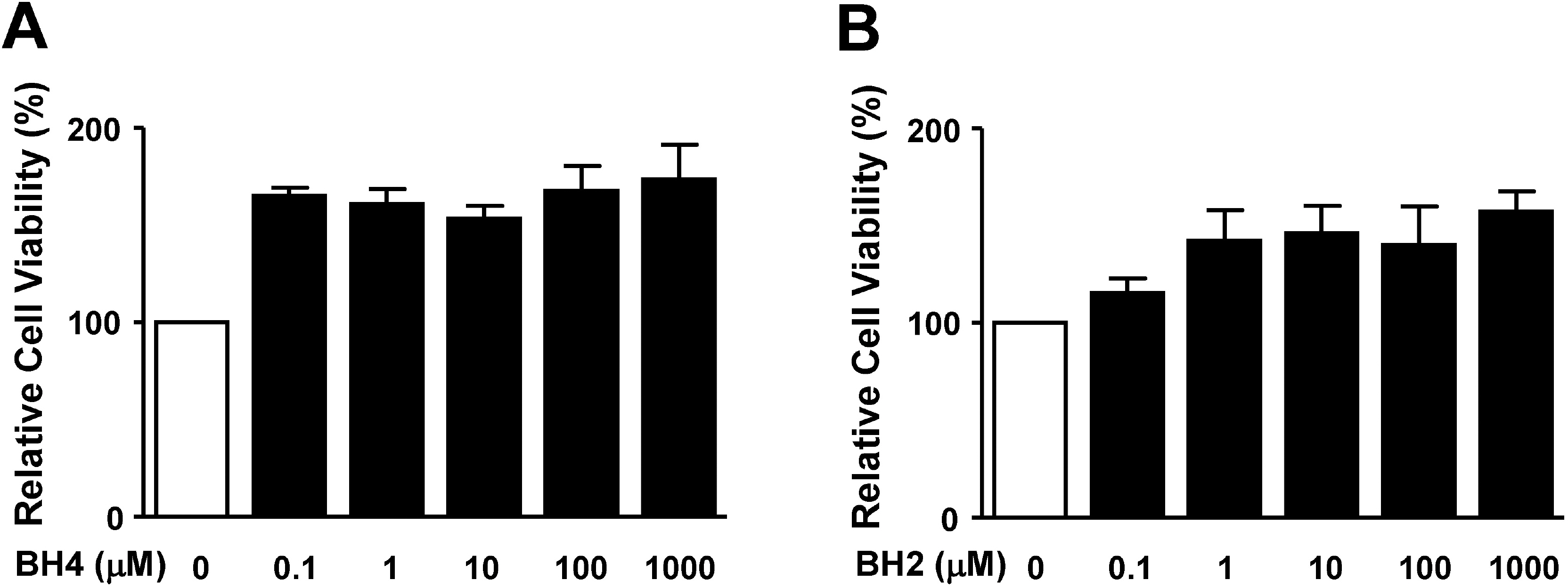
- Tetrahydrobiopterin (BH4), an essential cofactor for nitric oxide synthase (NOS) activity, is known to play important roles in modulating both NO and superoxide production during vascular diseases such as atherosclerosis. However, the role of BH4 in functions of vascular smooth muscle cells is not fully known. In this study, we tested the effects of BH4 and dihydrobiopterin (BH2), a BH4 precursor, on migration and proliferation in response to platelet-derived growth factor-BB (PDGF-BB) in rat aortic smooth muscle cells (RASMCs). Cell migration and proliferation were measured using a Boyden chamber and a 5-bromo-2'-deoxyuridine incorporation assay, respectively, and these results were confirmed with an ex vivo aortic sprout assay. Cell viability was examined by 2,3-bis [2-methoxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide assays. BH4 and BH2 decreased PDGF-BB-induced cell migration and proliferation in a dose-dependent manner. The inhibition of cell migration and proliferation by BH4 and BH2 was not affected by pretreatment with N G-nitro-L-arginine methyl ester, a NOS inhibitor. Moreover, the sprout outgrowth formation of aortic rings induced by PDGF-BB was inhibited by BH4 and BH2. Cell viability was not inhibited by BH4 and BH2 treatment. The present results suggest that BH4 and BH2 may inhibit PDGF-stimulated RASMC migration and proliferation via the NOS-independent pathway. Therefore, BH4 and its derivative could be useful for the development of a candidate molecule with an NO-independent anti-atherosclerotic function.
MeSH Terms
-
Animals
Atherosclerosis
Biopterin
Bromodeoxyuridine
Cell Movement
Cell Survival
Muscle, Smooth
Muscle, Smooth, Vascular
Myocytes, Smooth Muscle
Nitric Oxide
Nitric Oxide Synthase
Proto-Oncogene Proteins c-sis
Rats
Superoxides
Vascular Diseases
Biopterin
Bromodeoxyuridine
Nitric Oxide
Nitric Oxide Synthase
Proto-Oncogene Proteins c-sis
Superoxides
Figure
Reference
-
References
1. Nichol CA, Lee CL, Edelstein MP, Chao JY, Duch DS. Biosynthesis of tetrahydropterin by de novo and savage pathways in adrenal medulla extracts, mammalian cell cultures, and rat brain in vitro. Proc Natl Acad Sci USA. 1983; 80:1546–1550.2. Kwon NS, Nathan CF, Stuehr DJ. Reduced biopterin as a cofactor in the generation of nitrogen oxides by murine macrophages. J Biol Chem. 1989; 264:20496–20501.
Article3. Mayer B, Werner ER. In search of function for tetrahy-drobipterin in the biosynthesis of nitric oxide. Arch Pharmacol. 1995; 351:453–463.
Article4. Moens AL, Kass DA. Tetrahydrodiopterin and cardiovascular disease. Aterioscler Thromb Vasc Biol. 2006; 26:2439–2444.5. Zheng J, Yang X, Keith J, Christian H, Fing GD, Gerogory K, Imre K, Chen AF. Gene transfer of human guanosine 5′-triphosphate cyclohydrolase I restores vascular tetrahydrobiopterin level and endothelial function in low rennin hypertension. Circulation. 2003; 108:1238–1245.6. Yang S, Lee YJ, Kim J, Park S, Peris J, Laipis P, Park YS, Chung JHC, Oh SP. A murine model for human sepiapterin reductase deficiency. Am J Hum Genet. 2006; 78:575–587.7. Nicholas JA, Keith M. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Aterioscler Thromb Vas Biol. 2004; 24:413–420.8. Wang CH, Li SH, Weisel RD, Fedak PW, Hung A, Li RK, Rao V, Hyland K, Cherng WJ, Errett L, Leclerc Y, Bonneau D, Latter DA, Verma S. Tetrahydrobiopterin deficiency exaggerates intimal hyperplasia after vascular injury. Am J Physiol Regul Integr Comp Physiol. 2005; 289:R299–R304.
Article9. Ali ZA, Bursill CA, Douglas G, McNeill E, Papaspyridonos M, Tatham AL, Bendall JK, Akhtar AM, Alp NJ, Greaves DR, Channon KM. CCR2-mediated anti-inflammatory effects of endothelial tetrahydrobiopterin inhibit vascular injury-induced accelerated atherosclerosis. Circulation. 2008; 118:S71–S77.
Article10. Lee CK, Han JS, Won KJ, Jung SH, Park HJ, Lee HM, Kim J, Park YS, Kim HJ, Park PJ, Park TK, Kim B. Diminished expression of dihydropteridine reductase is a potent biomarker for hypertensive vessels. Proteomics. 2009; 9:4851–4858.
Article11. Lee HM, Jeon BH, Won KJ, Lee CK, Park TK, Choi WS, Bae YM, Kim HS, Lee SK, Park SH, Irani K, Kim B. Gene transfer of redox factor-1 inhibits neointimal formation: involvement of platelet-derived growth factor-beta receptor signaling via the inhibition of the reactive oxygen species-mediated Syk pathway. Circ Res. 2009; 104:219–227.12. Raines EW. PDGF and cardiovascular disease. Cytokine Growth Factor Rev. 2004; 15:237–254.
Article13. Lee CK, Lee HM, Kim HJ, Park HJ, Won KJ, Roh HY, Choi WS, Jeon BH, Park TK, Kim B. Syk contributes to PDGF-BB-mediated migration of rat aortic smooth muscle cells via MAPK pathways. Cardiovasc Res. 2007; 74:159–168.
Article14. Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999; 79:1283–1316.
Article15. Won KJ, Lee SC, Lee CK, Lee HM, Lee SH, Fang Z, Choi OB, Jin M, Kim J, Park T, Choi WS, Kim SK, Kim B. Cordycepin attenuates neointimal formation by inhibiting reactive oxygen species-mediated responses in vascular smooth muscle cells in rats. J Pharmacol Sci. 2009; 109:403–412.
Article16. Sachinidis A, Locher R, Vetter W, Tatje D, Hoppe J. Different effects of platelet-derived growth factor isoforms on rat vascular smooth muscle cells. J Biol Chem. 1990; 265:10238–10243.
Article17. Moens AL, Kass DA. Therapeutic potential of tetrahydrobiopterin for treating vascular and cardiac disease. J Cardiovasc Pharmacol. 2007; 50:238–246.
Article18. Anastasiadis PZ, Bezin L, Imerman BA, Kuhn DM, Louie MC, Levine RA. Tetrahydrobiopterin as a mediator of PC12 cell proliferation induced by EGF and NGF. Eur J Neurosci. 1997; 9:1831–1837.19. Shimizu S, Yasuda M, Ishii M, Nagai T, Kiuchi Y, Yamamoto T. Stimulation of in vitro angiogenesis by tetrahydrobiopterin in bovine aortic endothelial cells. Jpn J Pharmacol. 1999; 80:177–180.
Article20. Stein CS, Fabry Z, Murphy S, Hart MN, Stein CS, Fabry Z, Murphy S, Hart MN. Involvement of nitric oxide in IFN-γ-mediated reduction of microvessel smooth muscle cell proliferation. Mol Immunol. 1995; 32:965–973.
Article21. Sarkar R, Meinberg EG, Stanley JC, Gordon D, Webb RC. Nitric oxide reversibly inhibits the migration of cultured vascular smooth muscle cells. Circ Res. 1996; 78:225–230.
Article22. Lee HM, Lee CK, Lee SH, Roh HY, Bae YM, Lee KY, Lim J, Park PJ, Park TK, Lee YL, Won KJ, Kim B. p38 mitogen-activated protein kinase contributes to angiotensin II-stimulated migration of rat aortic smooth muscle cells. J Pharmacol Sci. 2007; 105:74–81.
Article23. Hasegawa H, Sawabe K, Nakanishi N, Wakasugi OK. Delivery of exogenous tetrahydrobiopterin (BH4) to cells of target organs: role of salvage pathway and uptake of its precursor in effective elevation of tissue BH4. Mol Genet Metab. 2005; 86:S2–S10.
Article24. Anastasiadis PZ, Jiang H, Bezin L, Kuhn DM, Levine RA. Tetrahydrobiopterin enhances apoptotic PC12 cell death following withdrawal of trophic support. J Biol Chem. 2001; 276:9050–9058.
Article25. Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994; 14:5147–5159.
Article26. Yetik-Anacak G, Catravas JD. Nitric oxide and the endothelium: history and impact on cardiovascular disease. Vascul Pharmacol. 2006; 45:268–276.
Article27. Puddu GM, Cravero E, Arnone G, Muscari A, Puddu P. Molecular aspects of atherogenesis: new insights and unsolved questions. J Biomed Sci. 2005; 12:839–853.
Article28. Katusic ZS. Vascular endothelial dysfunction: does tetrahydrobiopterin play a role? Am J Physiol Heart Circ Physiol. 2001; 281:H981–H986.
Article29. Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol. 2004; 24:413–420.
Article30. Nakaki T, Nakayama M, Kato R. Nakaki T, Nakayama M, Kato R. Inhibition by nitric oxide and nitric oxide-producing vasodilators of DNA synthesis in vascular smooth muscle cells. Eur J Pharmacol. 1990; 189:347–353.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Influence of Endothelin-1 on Cultured Vascular Smooth Muscle Cell Proliferation
- The Effects of Cilostazol on Proliferation of Vascular Smooth Muscle Cells and Expression of iNOS and p21
- Olibanum Extract Inhibits Vascular Smooth Muscle Cell Migration and Proliferation in Response to Platelet-Derived Growth Factor
- Effects of Nitric Oxide Synthase Inhibitor on Vascular Responses of Lidocaine and Bupivacaine in Endotoxemic Rats Aorta
- Expression of Constitutive Nitric Oxide Synthase by Gastrointestinal Epithelial Cells