Diabetes Metab J.
2011 Apr;35(2):149-158. 10.4093/dmj.2011.35.2.149.
Changes in Adenosine Deaminase Activity in Patients with Type 2 Diabetes Mellitus and Effect of DPP-4 Inhibitor Treatment on ADA Activity
- Affiliations
-
- 1Department of Internal Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea. Ldhkso@jejunu.ac.kr
- 2Department of Laboratory Medicine, Jeju National University Hospital, Jeju National University School of Medicine, Jeju, Korea.
- KMID: 2281713
- DOI: http://doi.org/10.4093/dmj.2011.35.2.149
Abstract
- BACKGROUND
Dipeptidyl peptidase 4 (DPP-4, also known as CD26) binds with adenosine deaminase (ADA) to activate T lymphocytes. Here, we investigated whether ADA activity is specifically affected by treatment with DPP-4 inhibitor (DPP4I) compared with other anti-diabetic agents.
METHODS
Fasting ADA activity, in addition to various metabolic and biochemical parameters, were measured in 262 type 2 diabetes mellitus (T2DM) patients taking various anti-diabetic agents and in 46 non-diabetic control subjects.
RESULTS
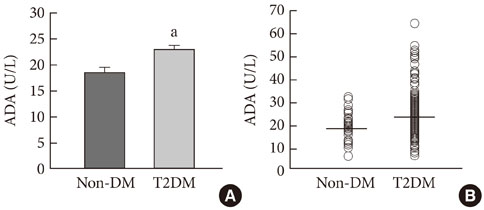
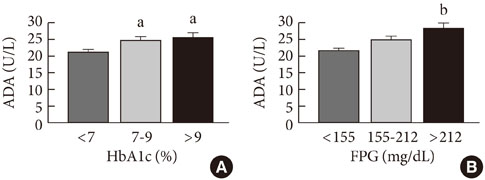
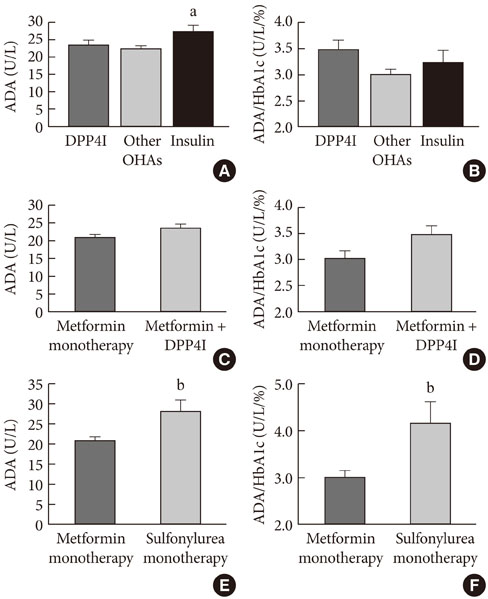
ADA activity was increased in T2DM patients compared with that in non-diabetic control subjects (mean+/-standard error, 23.1+/-0.6 U/L vs. 18.6+/-0.8 U/L; P<0.05). ADA activity was correlated with fasting plasma glucose (r=0.258, P<0.05), HbA1c (r=0.208, P<0.05), aspartate aminotransferase (r=0.325, P<0.05), and alanine aminotransferase (r=0.248, P<0.05). Compared with the well-controlled T2DM patients (HbA1c<7%), the poorly controlled group (HbA1c>9%) showed significantly increased ADA activity (21.1+/-0.8 U/L vs. 25.4+/-1.6 U/L; P<0.05). The effect of DPP4I on ADA activity in T2DM patients did not differ from those of other oral anti-diabetic agents or insulin. T2DM patients on metformin monotherapy showed a lower ADA activity (20.9+/-1.0 U/L vs. 28.1+/-2.8 U/L; P<0.05) compared with that of those on sulfonylurea monotherapy.
CONCLUSION
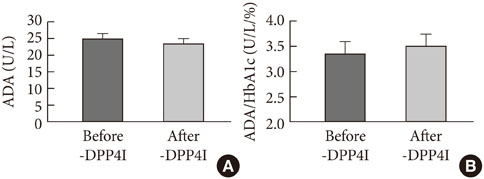
Our results show that ADA activity is increased in T2DM patients compared to that in non-diabetic patients, is positively correlated with blood glucose level, and that DPP4I has no additional specific effect on ADA activity, except for a glycemic control- or HbA1c-dependent effect.
MeSH Terms
-
Adenosine
Adenosine Deaminase
Alanine Transaminase
Aspartate Aminotransferases
Blood Glucose
Diabetes Mellitus, Type 2
Dipeptidyl Peptidase 4
Fasting
Glucose
Humans
Insulin
Metformin
Plasma
T-Lymphocytes
Adenosine
Adenosine Deaminase
Alanine Transaminase
Aspartate Aminotransferases
Blood Glucose
Dipeptidyl Peptidase 4
Glucose
Insulin
Metformin
Figure
Reference
-
1. Drucker DJ. Enhancing incretin action for the treatment of type 2 diabetes. Diabetes Care. 2003. 26:2929–2940.2. Ahren B. Gut peptides and type 2 diabetes mellitus treatment. Curr Diab Rep. 2003. 3:365–372.3. Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002. 359:824–830.4. Drucker DJ. Biological actions and therapeutic potential of the glucagon-like peptides. Gastroenterology. 2002. 122:531–544.5. Nauck MA, Niedereichholz U, Ettler R, Holst JJ, Orskov C, Ritzel R, Schmiegel WH. Glucagon-like peptide 1 inhibition of gastric emptying outweighs its insulinotropic effects in healthy humans. Am J Physiol. 1997. 273(5 Pt 1):E981–E988.6. Turton MD, O'Shea D, Gunn I, Beak SA, Edwards CM, Meeran K, Choi SJ, Taylor GM, Heath MM, Lambert PD, Wilding JP, Smith DM, Ghatei MA, Herbert J, Bloom SR. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature. 1996. 379:69–72.7. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995. 44:1126–1131.8. Lambeir AM, Durinx C, Scharpe S, De Meester I. Dipeptidyl-peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci. 2003. 40:209–294.9. Gorrell MD, Gysbers V, McCaughan GW. CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol. 2001. 54:249–264.10. De Meester I, Korom S, Van Damme J, Scharpe S. CD26, let it cut or cut it down. Immunol Today. 1999. 20:367–375.11. Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007. 298:194–206.12. Richter B, Bandeira-Echtler E, Bergerhoff K, Lerch CL. Dipeptidyl peptidase-4 (DPP-4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008. (2):CD006739.13. Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H. Sitagliptin Study 023 Group. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006. 49:2564–2571.14. Kirby M, Yu DM, O'Connor S, Gorrell MD. Inhibitor selectivity in the clinical application of dipeptidyl peptidase-4 inhibition. Clin Sci (Lond). 2009. 118:31–41.15. Martin DW Jr, Gelfand EW. Biochemistry of diseases of immunodevelopment. Annu Rev Biochem. 1981. 50:845–877.16. Ocana I, Martinez-Vazquez JM, Segura RM, Fernandez-De-Sevilla T, Capdevila JA. Adenosine deaminase in pleural fluids. Test for diagnosis of tuberculous pleural effusion. Chest. 1983. 84:51–53.17. Barnes PF, Mistry SD, Cooper CL, Pirmez C, Rea TH, Modlin RL. Compartmentalization of a CD4+ T lymphocyte subpopulation in tuberculous pleuritis. J Immunol. 1989. 142:1114–1119.18. Brady T. Adenosine deaminase. Biochem J. 1942. 36:478–484.19. Ratech H, Hirschhorn R. Serum adenosine deaminase in normals and in a patient with adenosine deaminase deficient-severe combined immunodeficiency. Clin Chim Acta. 1981. 115:341–347.20. Niedzwicki JG, Abernethy DR. Structure-activity relationship of ligands of human plasma adenosine deaminase2. Biochem Pharmacol. 1991. 41:1615–1624.21. Prakash MS, Chennaiah S, Murthy YS, Anjaiah E, Rao SA, Suresh C. Altered adenosine deaminase activity in type 2 diabetes mellitus. JIACM. 2006. 7:114–117.22. Mokhtari M, Hashemi M, Yaghmaei M, Molashahi F, Shikhzadeh A, Niazi A, Ghavami S. Serum adenosine deaminase activity in gestational diabetes mellitus and normal pregnancy. Arch Gynecol Obstet. 2010. 281:623–626.23. Kurtul N, Pence S, Akarsu E, Kocoglu H, Aksoy Y, Aksoy H. Adenosine deaminase activity in the serum of type 2 diabetic patients. Acta Medica (Hradec Kralove). 2004. 47:33–35.24. Kather H. Pathways of purine metabolism in human adipocytes. Further evidence against a role of adenosine as an endogenous regulator of human fat cell function. J Biol Chem. 1990. 265:96–102.25. Koopmans SJ, Sips HC, Bosman J, Radder JK, Krans HM. Antilipolytic action of insulin in adipocytes from starved and diabetic rats during adenosine-controlled incubations. Endocrinology. 1989. 125:3044–3050.26. Green A, Newsholme EA. Sensitivity of glucose uptake and lipolysis of white adipocytes of the rat to insulin and effects of some metabolites. Biochem J. 1979. 180:365–370.27. Kobayashi F, Ikeda T, Marumo F, Sato C. Adenosine deaminase isoenzymes in liver disease. Am J Gastroenterol. 1993. 88:266–271.28. Kalkan A, Bulut V, Erel O, Avci S, Bingol NK. Adenosine deaminase and guanosine deaminase activities in sera of patients with viral hepatitis. Mem Inst Oswaldo Cruz. 1999. 94:383–386.29. Lankas GR, Leiting B, Roy RS, Eiermann GJ, Beconi MG, Biftu T, Chan CC, Edmondson S, Feeney WP, He H, Ippolito DE, Kim D, Lyons KA, Ok HO, Patel RA, Petrov AN, Pryor KA, Qian X, Reigle L, Woods A, Wu JK, Zaller D, Zhang X, Zhu L, Weber AE, Thornberry NA. Dipeptidyl peptidase IV inhibition for the treatment of type 2 diabetes: potential importance of selectivity over dipeptidyl peptidases 8 and 9. Diabetes. 2005. 54:2988–2994.30. Prati D, Taioli E, Zanella A, Della Torre E, Butelli S, Del Vecchio E, Vianello L, Zanuso F, Mozzi F, Milani S, Conte D, Colombo M, Sirchia G. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann Intern Med. 2002. 137:1–10.31. Biftu T, Scapin G, Singh S, Feng D, Becker JW, Eiermann G, He H, Lyons K, Patel S, Petrov A, Sinha-Roy R, Zhang B, Wu J, Zhang X, Doss GA, Thornberry NA, Weber AE. Rational design of a novel, potent, and orally bioavailable cyclohexylamine DPP-4 inhibitor by application of molecular modeling and X-ray crystallography of sitagliptin. Bioorg Med Chem Lett. 2007. 17:3384–3387.32. Kim SJ, Nian C, McIntosh CH. Sitagliptin (MK0431) inhibition of dipeptidyl peptidase IV decreases nonobese diabetic mouse CD4+ T-cell migration through incretin-dependent and -independent pathways. Diabetes. 2010. 59:1739–1750.33. Paiva M, Riksen NP, Davidson SM, Hausenloy DJ, Monteiro P, Goncalves L, Providencia L, Rongen GA, Smits P, Mocanu MM, Yellon DM. Metformin prevents myocardial reperfusion injury by activating the adenosine receptor. J Cardiovasc Pharmacol. 2009. 53:373–378.34. Kowalczyk E, Kopff M, Kowalski J, Kopff A, Mikhailidis DP, Barylski M, Banach M. Effect of cardiovascular drugs on adenosine deaminase activity. Angiology. 2008-2009. 59:740–744.35. Albert MA, Danielson E, Rifai N, Ridker PM. PRINCE Investigators. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (PRINCE): a randomized trial and cohort study. JAMA. 2001. 286:64–70.36. Palinski W. Immunomodulation: a new role for statins? Nat Med. 2000. 6:1311–1312.37. Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000. 6:1399–1402.38. Hoshino T, Yamada K, Masuoka K, Tsuboi I, Itoh K, Nonaka K, Oizumi K. Elevated adenosine deaminase activity in the serum of patients with diabetes mellitus. Diabetes Res Clin Pract. 1994. 25:97–102.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adenosine Deaminase in Human Skin
- A study on the diagnostic value of cerebrospinal fluid adenosine deaminase activity in children with tuberculous meningitis
- Diagnostic Efficacy of Adenosine Deaminase Isoenzyme in Tuberculous Pleurisy
- Synovial Fluid Adenosine Deaminse Activity in the Patients of Rheumatoid Arthritis, Osteoarthritis, Ankylosing Spondylitis, and Gouty Arthritis
- The Utility of Pleural Adenosine Deaminase for Diagnosis of Differentiating Tuberculous Pleural Effusion in Children