Comparison of the Efficacy of Glimepiride, Metformin, and Rosiglitazone Monotherapy in Korean Drug-Naive Type 2 Diabetic Patients: The Practical Evidence of Antidiabetic Monotherapy Study
- Affiliations
-
- 1Department of Endocrinology, The Catholic University of Korea School of Medicine, Seoul, Korea. hys@catholic.ac.kr
- 2Department of Internal Medicine, Eulji University School of Medicine, Daejeon, Korea.
- 3Department of Endocrinology, Kyung Hee East-West Neo Medical Center, Seoul, Korea.
- 4Department of Internal Medicine, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Department of Endocrinology, Ajou University School of Medicine, Suwon, Korea.
- 6Department of Endocrinology, Ewha Woman's University School of Medicine, Seoul, Korea.
- 7Department of Internal Medicine, Chonbuk National University Hospital, Jeonju, Korea.
- 8Department of Internal Medicine, University of Ulsan College of Medicine, Seoul, Korea.
- 9Department of Internal Medicine, Pusan National University School of Medicine, Busan, Korea.
- 10Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea.
- 11Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea.
- 12Department of Endocrinology, Gachon University of Science & Medicine, Incheon, Korea.
- 13Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 14Department of Endocrinology, Kyung Hee University College of Medicine, Seoul, Korea.
- KMID: 2281489
- DOI: http://doi.org/10.4093/dmj.2011.35.1.26
Abstract
- BACKGROUND
Although many anti-diabetic drugs have been used to control hyperglycemia for decades, the efficacy of commonly-used oral glucose-lowering agents in Korean type 2 diabetic patients has yet to be clearly demonstrated.
METHODS
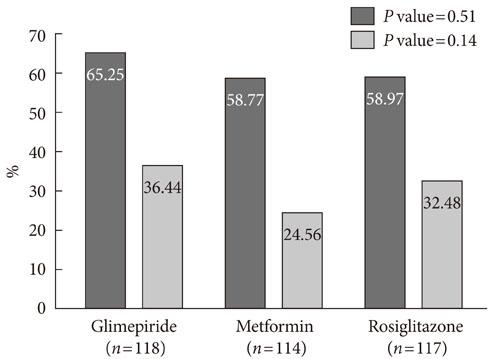
We evaluated the efficacy of glimepiride, metformin, and rosiglitazone as initial treatment for drug-naive type 2 diabetes mellitus patients in a 48-week, double-blind, randomized controlled study that included 349 Korean patients. Our primary goal was to determine the change in HbA1c levels from baseline to end point. Our secondary goal was to evaluate changes in fasting plasma glucose (FPG) levels, body weight, frequency of adverse events, and the proportion of participants achieving target HbA1c levels.
RESULTS
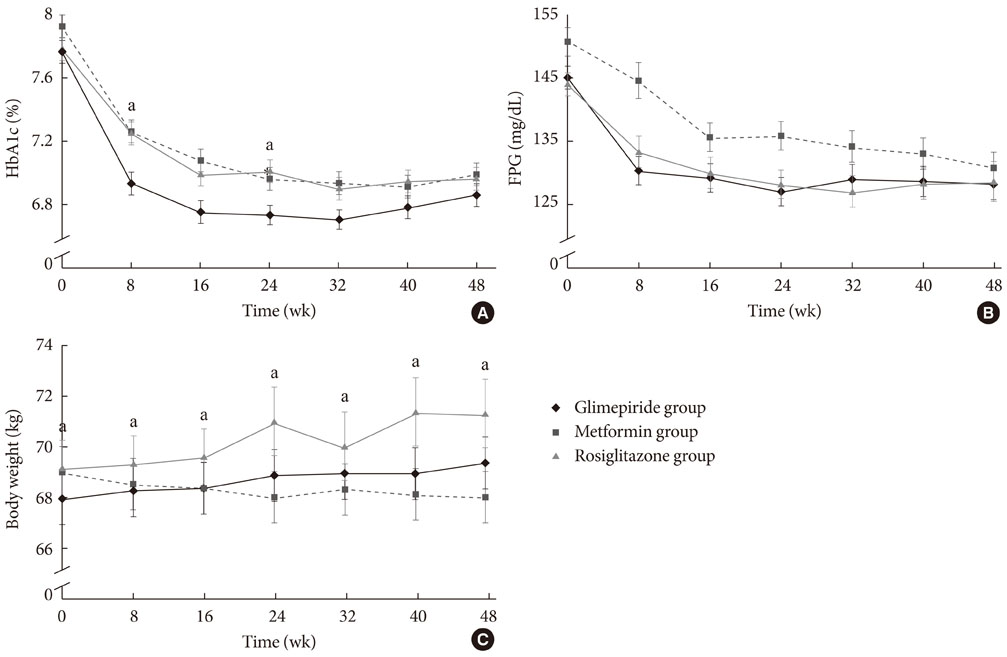
HbA1c levels decreased from 7.8% to 6.9% in the glimepiride group (P<0.001), from 7.9% to 7.0% in the metformin group (P<0.001), and from 7.8% to 7.0% (P<0.001) in the rosiglitazone group. Glimepiride and rosiglitazone significantly increased body weight and metformin reduced body weight during the study period. Symptomatic hypoglycemia was more frequent in the glimepiride group and diarrhea was more frequent in the metformin group.
CONCLUSION
The efficacy of glimepiride, metformin, and rosiglitazone as antidiabetic monotherapies in drug-naive Korean type 2 diabetic patients was similar in the three groups, with no statistical difference. This study is the first randomized controlled trial to evaluate the efficacy of commonly-used oral hypoglycemic agents in Korean type 2 diabetic patients. An additional subgroup analysis is recommended to obtain more detailed information.
MeSH Terms
Figure
Cited by 6 articles
-
Oral Hypoglycemic Agents for Patients with Type 2 Diabetes Mellitus
Seung-Hyun Ko
J Korean Diabetes. 2019;20(3):142-148. doi: 10.4093/jkd.2019.20.3.142.2019 Clinical Practice Guidelines for Type 2 Diabetes Mellitus in Korea
Mee Kyoung Kim, Seung-Hyun Ko, Bo-Yeon Kim, Eun Seok Kang, Junghyun Noh, Soo-Kyung Kim, Seok-O Park, Kyu Yeon Hur, Suk Chon, Min Kyong Moon, Nan-Hee Kim, Sang Yong Kim, Sang Youl Rhee, Kang-Woo Lee, Jae Hyeon Kim, Eun-Jung Rhee, SungWan Chun, Sung Hoon Yu, Dae Jung Kim, Hyuk-Sang Kwon, Kyong Soo Park,
Diabetes Metab J. 2019;43(4):398-406. doi: 10.4093/dmj.2019.0137.Clinical Practice Guideline 2015: Oral Hypoglycemic Agents for Patients with Type 2 Diabetes
Seung-Hyun Ko
J Korean Diabetes. 2016;17(2):83-87. doi: 10.4093/jkd.2016.17.2.83.Monotherapy in Type 2 Diabetes Mellitus Patients 2017: A Position Statement of the Korean Diabetes Association
Sang Youl Rhee
J Korean Diabetes. 2018;19(1):15-22. doi: 10.4093/jkd.2018.19.1.15.Insulin Resistance, Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus: Clinical and Experimental Perspective
Inha Jung, Dae-Jeong Koo, Won-Young Lee
Diabetes Metab J. 2024;48(3):327-339. doi: 10.4093/dmj.2023.0350.2023 Clinical Practice Guidelines for Diabetes Management in Korea: Full Version Recommendation of the Korean Diabetes Association
Jun Sung Moon, Shinae Kang, Jong Han Choi, Kyung Ae Lee, Joon Ho Moon, Suk Chon, Dae Jung Kim, Hyun Jin Kim, Ji A Seo, Mee Kyoung Kim, Jeong Hyun Lim, Yoon Ju Song, Ye Seul Yang, Jae Hyeon Kim, You-Bin Lee, Junghyun Noh, Kyu Yeon Hur, Jong Suk Park, Sang Youl Rhee, Hae Jin Kim, Hyun Min Kim, Jung Hae Ko, Nam Hoon Kim, Chong Hwa Kim, Jeeyun Ahn, Tae Jung Oh, Soo-Kyung Kim, Jaehyun Kim, Eugene Han, Sang-Man Jin, Jaehyun Bae, Eonju Jeon, Ji Min Kim, Seon Mee Kang, Jung Hwan Park, Jae-Seung Yun, Bong-Soo Cha, Min Kyong Moon, Byung-Wan Lee
Diabetes Metab J. 2024;48(4):546-708. doi: 10.4093/dmj.2024.0249.
Reference
-
1. Kim DJ, Lee MS, Kim KW, Lee MK. Insulin secretory dysfunction and insulin resistance in the pathogenesis of Korean type 2 diabetes mellitus. Metabolism. 2001. 50:590–593.2. Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006. 368:1681–1688.3. Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH, Hu FB. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009. 301:2129–2140.4. Global risks 2009: a global risk network report. World Economics Forum. 2009. In : World Economics Forum; Geneva.5. American Diabetes Association. Standards of medical care in diabetes--2007. Diabetes Care. 2007. 30:Suppl 1. S4–S41.6. Riddle MC. Oral pharmacologic management of type 2 diabetes. Am Fam Physician. 1999. 60:2613–2620.7. Bell DS. Type 2 diabetes mellitus: what is the optimal treatment regimen? Am J Med. 2004. 116:Suppl 5A. 23S–29S.8. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O'Neill MC, Zinman B, Viberti G. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006. 355:2427–2443.9. Yamanouchi T, Sakai T, Igarashi K, Ichiyanagi K, Watanabe H, Kawasaki T. Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed Type 2 diabetes. Diabet Med. 2005. 22:980–985.10. Task Force Team of Korean Diabetes Association. Treatment guideline for diabetes. 2007. 1st ed. Seoul: Korean Diabetes Association;48–58.11. Cheng AY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005. 172:213–226.12. Hoffmann J, Spengler M. Efficacy of 24-week monotherapy with acarbose, metformin, or placebo in dietary-treated NIDDM patients: the Essen-II Study. Am J Med. 1997. 103:483–490.13. Lebovitz HE, Dole JF, Patwardhan R, Rappaport EB, Freed MI. Rosiglitazone Clinical Trials Study Group. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J Clin Endocrinol Metab. 2001. 86:280–288.14. Tan M, Johns D, Gonzalez Galvez G, Antunez O, Fabian G, Flores-Lozano F, Zuniga Guajardo S, Garza E, Morales H, Konkoy C, Herz M. GLAD Study Group. Effects of pioglitazone and glimepiride on glycemic control and insulin sensitivity in Mexican patients with type 2 diabetes mellitus: a multicenter, randomized, double-blind, parallel-group trial. Clin Ther. 2004. 26:680–693.15. Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin: therapeutic and cellular mechanisms. Drugs. 1999. 58:Suppl 1. 31–39.16. Davis SN. The role of glimepiride in the effective management of type 2 diabetes. J Diabetes Complications. 2004. 18:367–376.17. Meriden T. Progress with thiazolidinediones in the management of type 2 diabetes mellitus. Clin Ther. 2004. 26:177–190.18. Rendell M. The role of sulphonylureas in the management of type 2 diabetes mellitus. Drugs. 2004. 64:1339–1358.19. Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE. Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N Engl J Med. 1995. 333:550–554.20. Lebovitz HE. Differentiating members of the thiazolidinedione class: a focus on safety. Diabetes Metab Res Rev. 2002. 18:Suppl 2. S23–S29.21. Sohn TS, Lee JI, Kim IJ, Min KW, Son HS. The effect of rosiglitazone and metformin therapy, as an initial therapy, in patients with type 2 diabetes mellitus. Korean Diabetes J. 2008. 32:445–452.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- What Is the Optimal Monotherapy in Korean Drug-Naive Type 2 Diabetic Patients?: The Practical Evidence of Antidiabetic Monotherapy Study
- Letter: Dipeptidyl Peptidase-4 Inhibitors versus Other Antidiabetic Drugs Added to Metformin Monotherapy in Diabetic Retinopathy Progression: A Real World-Based Cohort Study (Diabetes Metab J 2019;43:640–8)
- DPP-4 Inhibitors and the Relations between Rosiglitazone and the Risk of Myocardial Infarction
- The Effect of Metformin in Obese Pediatric Patients with Type 2 Diabetes
- Antihyperglycemic agent combination therapy for patients with type 2 diabetes mellitus