Clin Exp Otorhinolaryngol.
2008 Dec;1(4):211-216.
Early Sensorineural Hearing Loss in Ob/Ob Mouse, an Animal Model of Type 2 Diabetes
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Bundang Jesaeng Hospital, Daejin Medical Center, Seongnam, Korea.
- 2Department of Otorhinolaryngology-Head and Neck Surgery, Sungkyunkwan University School of Medicine, Samsung Medical Center, Seoul, Korea. hongsh@skku.edu
Abstract
OBJECTIVES
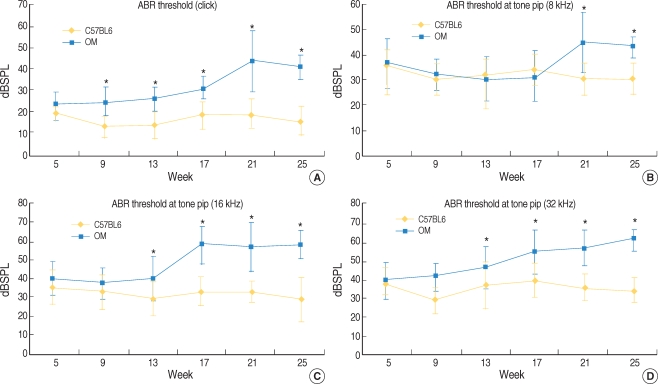
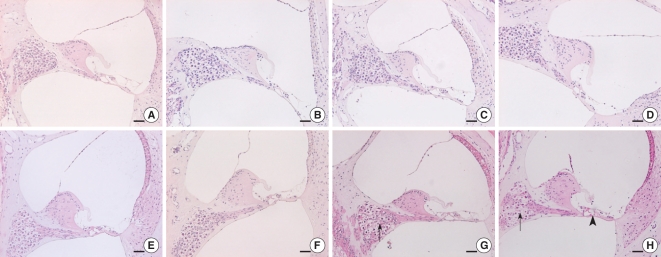
There have been many studies on the relationship between diabetes mellitus and presbycusis. Microangiopathy and neuropathy that's caused by chronic hyperglycemia may lead to damage to the inner ear. Several clinical studies on humans and animal studies have been performed to investigate the association between diabetes and hearing loss, however, this relationship is still a matter of debate. We investigated the association of diabetes and sensorineural hearing loss in an animal model of type-2 diabetes and obesity (the ob/ob mouse [OM]). METHODS: The auditory brainstem response (ABR) thresholds were obtained in the OM and the wild type mice (C57BL/6J mice) up to 25 weeks after birth. After the animals were sacrificed, their cochleae were retrieved and then subjected to histopathologic observations. RESULTS: The OM exhibited significantly elevated ABR thresholds at 21 weeks of age, yet the C57BL/6J mice exhibited no significant change until 25 weeks of age. On the histological findings, outer hair cell degeneration and loss of spiral ganglion cells were observed in the middle and basal turns of the OM. On the contrary, no degenerative change was observed until 25 weeks of age in the C57BL/6J mice. CONCLUSION: This study suggests that chronic hyperglycemia and obesity may lead to early sensorineural hearing loss.
Keyword
MeSH Terms
Figure
Reference
-
1. Cullen JR, Cinnamond MJ. Hearing loss in diabetics. J Laryngol Otol. 1993; 3. 107(3):179–182. PMID: 8509689.
Article2. Dalton DS, Cruickshanks KJ, Klein R, Klein BE, Wiley TL. Association of NIDDM and hearing loss. Diabetes Care. 1998; 9. 21(9):1540–1544. PMID: 9727906.
Article3. Ferrer JP, Biurrun O, Lorente J, Conget JI, dem Espaxa R, Esmatjes E, et al. Auditory function in young patients with type 1 diabetes mellitus. Diabetes Res Clin Pract. 1991; 1. 11(1):17–22. PMID: 2019230.
Article4. Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Characterization of hearing loss in aged type II diabetics. Hear Res. 2006; 1. 211(1-2):103–113. PMID: 16309862.
Article5. Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol. 2003; 5. 24(3):382–386. PMID: 12806288.
Article6. Tay HL, Ray N, Ohri R, Frootko NJ. Diabetes mellitus and hearing loss. Clin Otolaryngol Allied Sci. 1995; 4. 20(2):130–134. PMID: 7634518.
Article7. Díaz de León-Morales LV, Jáuregui-Renaud K, Garay-Sevilla ME, Hernández-Prado J, Malacara-Hernández JM. Auditory impairment in patients with type 2 diabetes mellitus. Arch Med Res. 2005; Sep–Oct. 36(5):507–510. PMID: 16099330.8. Sakuta H, Suzuki T, Yasuda H, Ito T. Type 2 diabetes and hearing loss in personnel of the Self-Defense Forces. Diabetes Res Clin Pract. 2007; 2. 75(2):229–234. PMID: 16963152.
Article9. de Espana R, Biurrun O, Lorente J, Traserra J. Hearing and diabetes. ORL J Otorhinolaryngol Relat Spec. 1995; Nov–Dec. 57(6):325–327. PMID: 8789482.10. Miller JJ, Beck L, Davis A, Jones DE, Thomas AB. Hearing loss in patients with diabetic retinopathy. Am J Otolaryngol. 1983; Sep–Oct. 4(5):342–346. PMID: 6638325.
Article11. Strauss P, Schneider K, Terriuolo V, Sachsse B. Inner ear and diabetes mellitus studies on 660 patients. Laryngol Rhinol Otol (Stuttg). 1982; 6. 61(6):331–338. PMID: 7121153.12. Friedman SA, Schulman RH, Weiss S. Hearing and diabetic neuropathy. Arch Intern Med. 1975; 4. 135(4):573–576. PMID: 1138672.
Article13. Taylor IG, Irwin J. Some audiological aspects of diabetes mellitus. J Laryngol Otol. 1978; 2. 92(2):99–113. PMID: 627773.
Article14. Jorgensen MB, Buch NH. Studies on inner-ear function and cranial nerves in diabetics. Acta Otolaryngol. 1961; May–Jun. 53:350–364. PMID: 13790713.15. Triana RJ, Suits GW, Garrison S, Prazma J, Brechtelsbauer PB, Michaelis OE, et al. Inner ear damage secondary to diabetes mellitus. I. Changes in adolescent SHR/N-cp rats. Arch Otolaryngol Head Neck Surg. 1991; 6. 117(6):635–640. PMID: 2036185.
Article16. McQueen CT, Baxter A, Smith TL, Raynor E, Yoon SM, Prazma J, et al. Non-insulin-dependent diabetic microangiopathy in the inner ear. J Laryngol Otol. 1999; 1. 113(1):13–18. PMID: 10341912.
Article17. Lindström P. The physiology of obese-hyperglycemic mice [ob/ob mice]. ScientificWorldJournal. 2007; 5. 7:666–685. PMID: 17619751.18. Nageris B, Hadar T, Feinmesser M, Elidan J. Cochlear histopathologic analysis in diabetic rats. Am J Otol. 1998; 1. 19(1):63–65. PMID: 9455951.19. Vaughan N, James K, McDermott D, Griest S, Fausti S. A 5-year prospective study of diabetes and hearing loss in a veteran population. Otol Neurotol. 2006; 1. 27(1):37–43. PMID: 16371845.
Article20. Fukushima H, Cureoglu S, Schachern PA, Paparella MM, Harada T, Oktay MF. Effects of type 2 diabetes mellitus on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006; 9. 132(9):934–938. PMID: 16982969.
Article21. Wackym PA, Linthicum FH Jr. Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol. 1986; 5. 7(3):176–182. PMID: 3717308.22. Smith TL, Raynor E, Prazma J, Buenting JE, Pillsbury HC. Insulin-dependent diabetic microangiopathy in the inner ear. Laryngoscope. 1995; 3. 105(3 Pt 1):236–240. PMID: 7877409.
Article23. Raynor EM, Carrasco VN, Prazma J, Pillsbury HC. An assessment of cochlear hair-cell loss in insulin-dependent diabetes mellitus diabetic and noise-exposed rats. Arch Otolaryngol Head Neck Surg. 1995; 4. 121(4):452–456. PMID: 7702821.
Article24. Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes and insulin resistance in ob/ob mice. Endocrinology. 2004; 7. 145(7):3258–3264. PMID: 15059949.
Article25. Ishikawa T, Naito Y, Taniguchi K. Hearing impairment in WBN/Kob rats with spontaneous diabetes mellitus. Diabetologia. 1995; 6. 38(6):649–655. PMID: 7672484.
Article26. Song L, McGee JA, Walsh EJ. Consequences of combined maternal, fetal and persistent postnatal hypothyroidism on the development of auditory function in Tshrhyt mutant mice. Brain Res. 2006; 7. 26. 1101(1):59–72. PMID: 16780814.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Inner Ear Gene Therapy in Mouse Models of Genetic Hearing Loss
- Morphological Study of the Mouse Inner Ear
- Retroviral - mediated Transduction of Leptin Gene in Genetically Obese Mice
- Rehabilitation of Sensorineural Hearing Loss: Hearing Aid
- Effects of Cyclo-His-Pro-enriched yeast hydrolysate on blood glucose levels and lipid metabolism in obese diabetic ob/ob mice