Nutr Res Pract.
2016 Apr;10(2):154-160. 10.4162/nrp.2016.10.2.154.
Effects of Cyclo-His-Pro-enriched yeast hydrolysate on blood glucose levels and lipid metabolism in obese diabetic ob/ob mice
- Affiliations
-
- 1Department of Home Economic Education, Jeonju University, Jeonju 55069, Korea.
- 2Department of Beauty Art, Suwon Women's University, Suwon 16632, Korea.
- 3LINC Project Group, Daejeon University, Daejeon 34520, Korea.
- 4Department of Public Health Sciences, Graduate School, Korea University, 145 Anam-ro, Sungbuk-gu, Seoul 02841, Korea. suh1960@korea.ac.kr
- KMID: 2313912
- DOI: http://doi.org/10.4162/nrp.2016.10.2.154
Abstract
- BACKGROUND/OBJECTIVES
We examined the hypoglycemic and anti-hyperlipidemic effect of yeast hydrolysate (YH) enriched with Cyclo-His-Pro (CHP) in the C57BL/6J ob/ob mouse model.
MATERIALS/METHODS
Mice were separated into 4 groups (8 mice/group) on the basis of blood glucose and body weight: WT control, lean mice given vehicle; ob/ob control, ob/ob mice given vehicle; YH-1, ob/ob mice given 0.5 g/kg of YH; YH-2, ob/ob mice given 1 g/kg of YH. YH in saline or vehicle was administered orally in the same volume every day for 3 weeks.
RESULTS
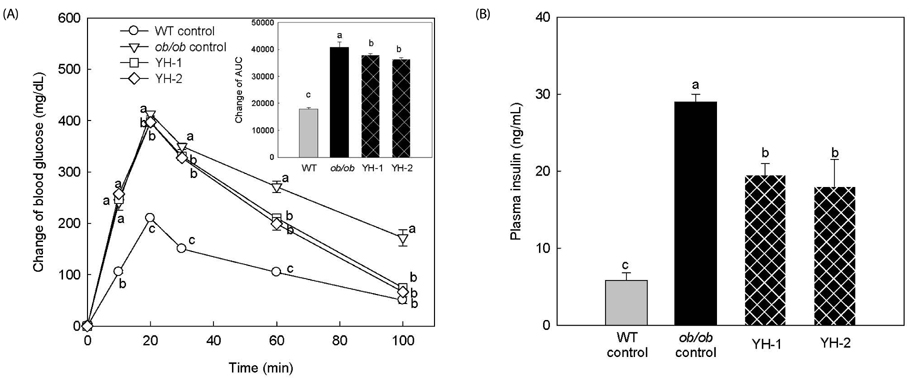
Mice treated with YH (0.5 and 1 g/kg) for 3 weeks displayed a significant reduction in overall body weight gain and perirenal and epididymal adipose tissue weight compared to the ob/ob control group. Additionally, high-density lipoprotein (HDL) cholesterol, glucose, and atherogenic indexes were significantly decreased in the blood of YH-1 and YH-2 groups compared to the ob/ob control. In ob/ob mice, YH administration significantly improved glucose tolerance and blood insulin levels. These data indicate that YH treatment produces potent hypoglycemic and anti-hyperlipidemic effects by controlling body weight, fat mass, blood lipid, insulin levels, and glucose tolerance.
CONCLUSION
YH could potentially be used as a treatment option for diabetes and hyperlipidemia. The CHP-enriched YH may be a promising strategy in the development of hypoglycemic peptide nutraceuticals.
MeSH Terms
Figure
Reference
-
1. Almdal T, Scharling H, Jensen JS, Vestergaard H. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004; 164:1422–1426.
Article2. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004; 27:2568–2569.
Article3. Goldfrank L, Lewin N, Flomenbaum N, Howland MA. The pernicious panacea: herbal medicine. Hosp Physician. 1982; 18:64–69.4. Sabu MC, Smitha K, Kuttan R. Anti-diabetic activity of green tea polyphenols and their role in reducing oxidative stress in experimental diabetes. J Ethnopharmacol. 2002; 83:109–116.
Article5. Pari L, Umamaheswari J. Antihyperglycaemic activity of Musa sapientum flowers: effect on lipid peroxidation in alloxan diabetic rats. Phytother Res. 2000; 14:136–138.
Article6. Tiwari AK, Rao JM. Diabetes mellitus and multiple therapeutic approaches of phytochemicals: present status and future prospects. Curr Sci. 2002; 83:30–38.7. Jung EY, Lee HS, Choi JW, Ra KS, Kim MR, Suh HJ. Glucose tolerance and antioxidant activity of spent brewer's yeast hydrolysate with a high content of Cyclo-His-Pro (CHP). J Food Sci. 2011; 76:C272–C278.
Article8. Hilton CW, Prasad C, Vo P, Mouton C. Food contains the bioactive peptide, cyclo(His-Pro). J Clin Endocrinol Metab. 1992; 75:375–378.
Article9. Hwang IK, Go VL, Harris DM, Yip I, Kang KW, Song MK. Effects of cyclo (his-pro) plus zinc on glucose metabolism in genetically diabetic obese mice. Diabetes Obes Metab. 2003; 5:317–324.
Article10. Song MK, Hwang IK, Rosenthal MJ, Harris DM, Yamaguchi DT, Yip I, Go VL. Anti-hyperglycemic activity of zinc plus cyclo (his-pro) in genetically diabetic Goto-Kakizaki and aged rats. Exp Biol Med (Maywood). 2003; 228:1338–1345.
Article11. Song MK, Rosenthal MJ, Song AM, Yang H, Ao Y, Yamaguchi DT. Raw vegetable food containing high cyclo (his-pro) improved insulin sensitivity and body weight control. Metabolism. 2005; 54:1480–1489.
Article12. Morley JE, Levine AS, Prasad C. Histidyl-proline diketopiperazine decreases food intake in rats. Brain Res. 1981; 210:475–478.
Article13. Steiner H, Wilber JF, Prasad C, Rogers D, Rosenkranz RT. Histidyl proline diketopiperazine (Cyclo [His-Pro]) in eating disorders. Neuropeptides. 1989; 14:185–189.
Article14. Song MK, Rosenthal MJ, Song AM, Uyemura K, Yang H, Ament ME, Yamaguchi DT, Cornford EM. Body weight reduction in rats by oral treatment with zinc plus cyclo-(His-Pro). Br J Pharmacol. 2009; 158:442–450.
Article15. Jung EY, Suh HJ, Kim SY, Hong YS, Kim MJ, Chang UJ. Appetite suppressive effects of yeast hydrolysate on nitric oxide synthase (NOS) expression and vasoactive intestinal peptide (VIP) immunoreactivity in hypothalamus. Phytother Res. 2008; 22:1417–1422.
Article16. Jung EY, Lee HS, Chang UJ, Bae SH, Kwon KH, Suh HJ. Acute and subacute toxicity of yeast hydrolysate from Saccharomyces cerevisiae. Food Chem Toxicol. 2010; 48:1677–1681.
Article17. Kim KM, Chang UJ, Kang DH, Kim JM, Choi YM, Suh HJ. Yeast hydrolysate reduces body fat of dietary obese rats. Phytother Res. 2004; 18:950–953.
Article18. Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. Evaluation of organ weights for rodent and non-rodent toxicity studies: a review of regulatory guidelines and a survey of current practices. Toxicol Pathol. 2007; 35:742–750.
Article19. Peters JM, Boyd EM. Organ weights and water levels of the rat following reduced food intake. J Nutr. 1966; 90:354–360.
Article20. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502.
Article21. Barham D, Trinder P. An improved colour reagent for the determination of blood glucose by the oxidase system. Analyst. 1972; 97:142–145.
Article22. Han GC, Ko SK, Sung JH, Chung SH. Compound K enhances insulin secretion with beneficial metabolic effects in db/db mice. J Agric Food Chem. 2007; 55:10641–10648.
Article23. Kim MJ, Chang UJ, Chung JH, Kim HK, Lim BO, Yamada K, Lim Y, Kang SA. Dissimilarity in Fos and Jun immunoreactivity in hypothalamic regions between obese and lean Zucker rats. Biosci Biotechnol Biochem. 2005; 69:1982–1984.
Article24. Arner P. Human fat cell lipolysis: biochemistry, regulation and clinical role. Best Pract Res Clin Endocrinol Metab. 2005; 19:471–482.
Article25. Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010; 316:129–139.
Article26. Lafontan M, Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res. 2009; 48:275–297.
Article27. Lin HZ, Yang SQ, Chuckaree C, Kuhajda F, Ronnet G, Diehl AM. Metformin reverses fatty liver disease in obese, leptin-deficient mice. Nat Med. 2000; 6:998–1003.
Article28. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern Med. 1999; 131:281–303.
Article29. Marles RJ, Farnsworth NR. Plants as sources of antidiabetic agents. In : Wagner H, Farnsworth NR, editors. Economic and Medicinal Plant Research. Vol. 6. London: Academic Press;1994. p. 149–188.30. Lee J, Chae K, Ha J, Park BY, Lee HS, Jeong S, Kim MY, Yoon M. Regulation of obesity and lipid disorders by herbal extracts from Morus alba, Melissa officinalis, and Artemisia capillaris in high-fat diet-induced obese mice. J Ethnopharmacol. 2008; 115:263–270.
Article31. den Boer M, Voshol PJ, Kuipers F, Havekes LM, Romijn JA. Hepatic steatosis: a mediator of the metabolic syndrome. Lessons from animal models. Arterioscler Thromb Vasc Biol. 2004; 24:644–649.
Article32. Arsenault BJ, Boekholdt SM, Kastelein JJ. Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol. 2011; 8:197–206.
Article33. Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002; 161:1–16.
Article34. Reaven GM. Insulin resistance in noninsulin-dependent diabetes mellitus. Does it exist and can it be measured? Am J Med. 1983; 74:3–17.35. Genuth SM, Przybylski RJ, Rosenberg DM. Insulin resistance in genetically obese, hyperglycemic mice. Endocrinology. 1971; 88:1230–1238.
Article36. Friedman JE, Dohm GL, Leggett-Frazier N, Elton CW, Tapscott EB, Pories WP, Caro JF. Restoration of insulin responsiveness in skeletal muscle of morbidly obese patients after weight loss. Effect on muscle glucose transport and glucose transporter GLUT4. J Clin Invest. 1992; 89:701–705.
Article37. Klein R, Klein BE, Moss SE, Cruickshanks KJ. Relationship of hyperglycemia to the long-term incidence and progression of diabetic retinopathy. Arch Intern Med. 1994; 154:2169–2178.
Article38. Liu QZ, Pettitt DJ, Hanson RL, Charles MA, Klein R, Bennett PH, Knowler WC. Glycated haemoglobin, plasma glucose and diabetic retinopathy: cross-sectional and prospective analyses. Diabetologia. 1993; 36:428–432.
Article39. Abraira C, Colwell JA, Nuttall FQ, Sawin CT, Nagel NJ, Comstock JP, Emanuele NV, Levin SR, Henderson W, Lee HS. Veterans Affairs Cooperative Study on glycemic control and complications in type II diabetes (VA CSDM). Results of the feasibility trial. Veterans Affairs Cooperative Study in Type II Diabetes. Diabetes Care. 1995; 18:1113–1123.
Article40. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.
Article41. Song MK, Rosenthal MJ, Hong S, Harris DM, Hwang I, Yip I, Golub MS, Ament ME, Go VL. Synergistic antidiabetic activities of zinc, cyclo (his-pro), and arachidonic acid. Metabolism. 2001; 50:53–59.
Article42. Rosenthal MJ, Hwang IK, Song MK. Effects of arachidonic acid and cyclo (his-pro) on zinc transport across small intestine and muscle tissues. Life Sci. 2001; 70:337–348.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Increased Plasma Dipeptidyl Peptidase IV Activities in ob/ob Mice
- Effect of sweet pumpkin powder on lipid metabolism in leptin-deficient mice
- Cordyceps militaris alleviates non-alcoholic fatty liver disease in ob/ob mice
- Anti-obesity activity of diglyceride containing conjugated linoleic acid in C57BL/6J ob/ob mice
- Retroviral - mediated Transduction of Leptin Gene in Genetically Obese Mice