Korean J Obstet Gynecol.
2010 Mar;53(3):243-253. 10.5468/kjog.2010.53.3.243.
Anti-proliferative effect in epithelial ovarian cancer cells by MEK Inhibitor AZD6244
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Pusan National University School of Medicine, Busan, Korea. ghkim@pusan.ac.kr
- KMID: 2273850
- DOI: http://doi.org/10.5468/kjog.2010.53.3.243
Abstract
OBJECTIVE
The objectives of this study were to determine the efficacy of AZD6244, a potent, selective MEK inhibitor, in epithelial ovarian cancer (EOC) cells and to determine the enhanced cell death by combined treatment of paclitaxel and AZD6244.
METHODS
EOC cells were treated with tenfold dilutions of AZD6244 (0.1 to 10 micrometer) for 24, 48 and 72 hours. Cell viability was determined by the CellTiter 96 AQueous One Solution Cell Proliferation Assay. The apoptotic cascade was assessed by Caspase-Glo assays. ERK activation was evaluated by Western blot analyses. Cytokine profiling was performed from culture supernatants using the Luminex 200 system.
RESULTS
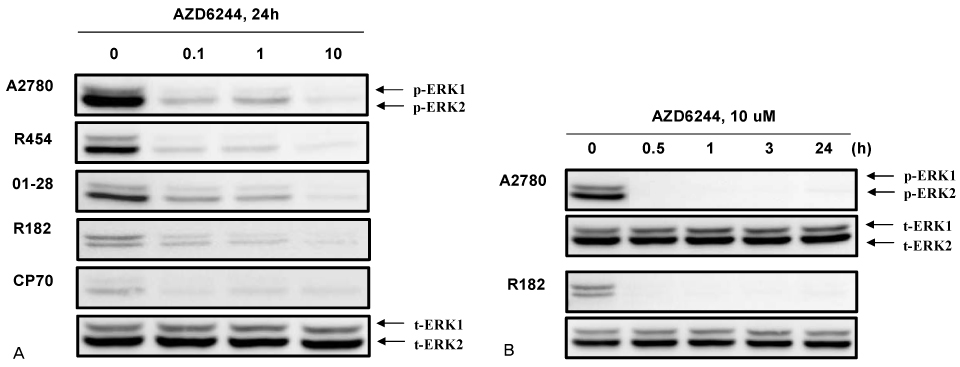
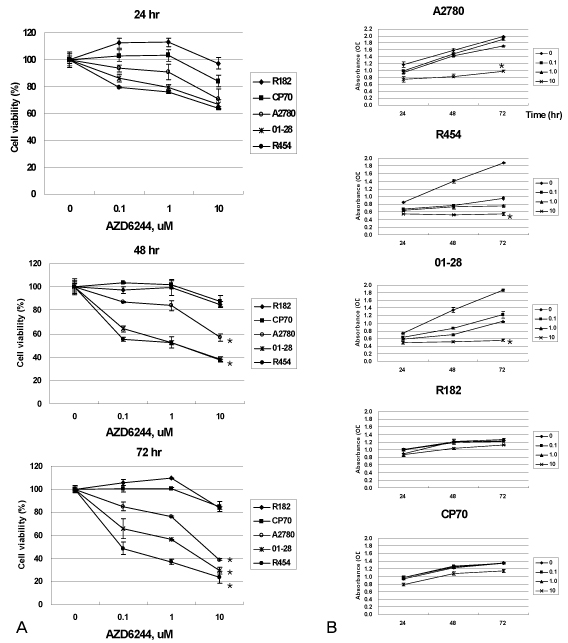
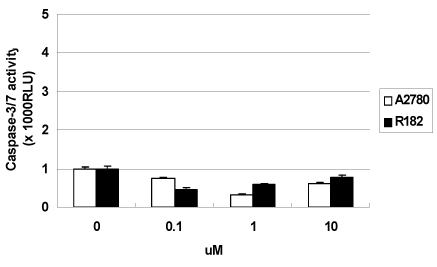
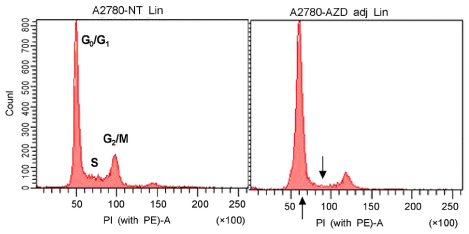
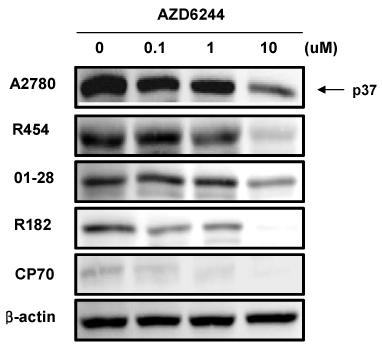
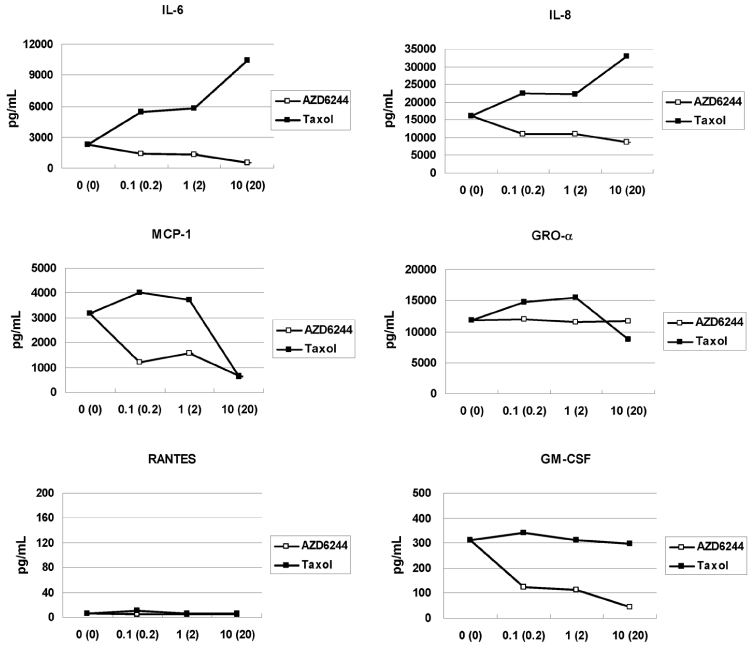
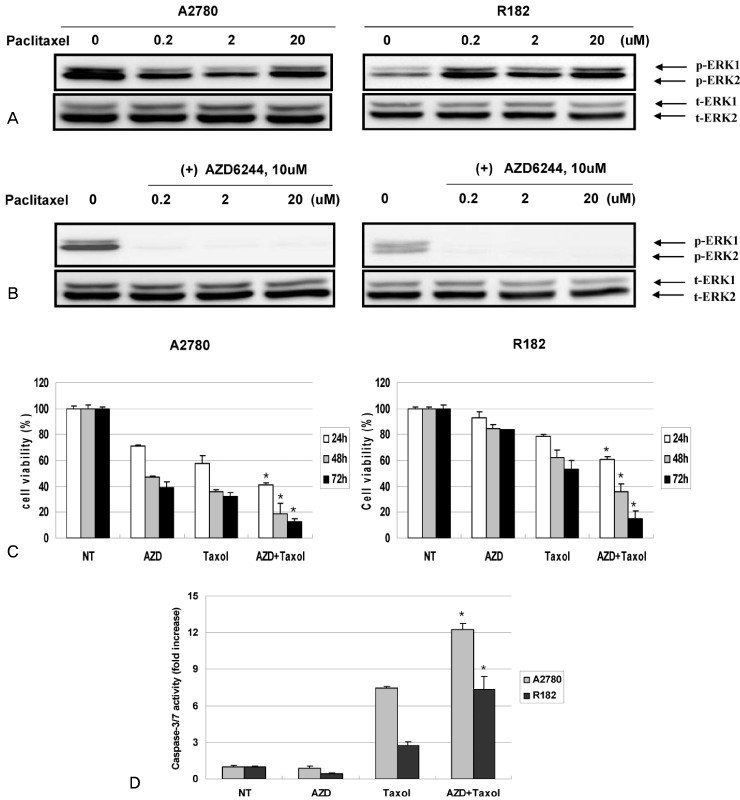
In vitro cell viability showed that ovarian cancer cells with high p-ERK activities (A2780, R454, 01-28) exhibited significant growth inhibition. Cells with low p-ERK activities (R182, CP70), however, were resistant to AZD6244. Caspase-3 was not activated during AZD6244-induced growth inhibition. AZD6244 significantly inhibited p-ERK1/2 in both cell types regardless of constitutive levels of p-ERK. Proinflammatory cytokines including IL-6, IL-8, MCP-1 and GM-CSF were significantly decreased. Paclitaxel activated the p-ERK levels in paclitaxel-resistant R182 cells with low basal p-ERK level. MEK inhibition by AZD6244 enhanced paclitaxel-induced apoptosis significantly in R182 cells.
CONCLUSION
These results demonstrate that AZD6244 has a potent growth inhibitory effect in ovarian cancer cells with high p-ERK activities. In addition, targeted inhibition of the extracellular signal-regulated kinase pathway with AZD6244 can enhance the anti-tumor efficacy of the cytotoxic paclitaxel.
Keyword
MeSH Terms
-
Apoptosis
Benzimidazoles
Blotting, Western
Caspase 3
Cell Death
Cell Proliferation
Cell Survival
Cytokines
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-6
Interleukin-8
Neoplasms, Glandular and Epithelial
Ovarian Neoplasms
Paclitaxel
Phosphotransferases
Benzimidazoles
Caspase 3
Cytokines
Granulocyte-Macrophage Colony-Stimulating Factor
Interleukin-6
Interleukin-8
Neoplasms, Glandular and Epithelial
Ovarian Neoplasms
Paclitaxel
Phosphotransferases
Figure
Reference
-
1. Aletti GD, Gallenberg MM, Cliby WA, Jatoi A, Hartmann LC. Current management strategies for ovarian cancer. Mayo Clin Proc. 2007. 82:751–770.
Article2. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008. 58:71–96.
Article3. Pouysségur J, Lenormand P. Fidelity and Spatio-temporal control in MAP kinase (ERKs) signaling. Eur J Biochem. 2003. 270:3291–3299.4. Friday BB, Yu C, Dy GK, Smith PD, Wang L, Thibodeau SN, et al. BRAF V600E disrupts AZD6244-induced abrogation of negative feedback pathways between extracellular signal-regulated kinase and Raf proteins. Cancer Res. 2008. 68:6145–6153.
Article5. Montagut C, Settleman J. Targeting the RAFMEK-ERK pathway in cancer therapy. Cancer Lett. 2009. 283:125–134.
Article6. Beeram M, Patnaik A, Rowinsky EK. Raf: a strategic target for therapeutic development against cancer. J Clin Oncol. 2005. 23:6771–6790.
Article7. Allen LF, Sebolt-Leopold J, Meyer MB. CI-1040 (PD184352), a targeted signal transduction inhibitor of MEK (MAPKK). Semin Oncol. 2003. 30:5 suppl 16. 105–116.
Article8. Zeng P, Wagoner HA, Pescovitz OH, Steinmetz R. RNA interference (RNAi) for extracellular signal-regulated kinase 1 (ERK1) alone is sufficient to suppress cell viability in ovarian cancer cells. Cancer Biol Ther. 2005. 4:961–967.
Article9. Hsu CY, Bristow R, Cha MS, Wang BG, Ho CL, Kurman RJ, et al. Characterization of active mitogen-activated protein kinase in ovarian serous carcinomas. Clin Cancer Res. 2004. 10:6432–6436.
Article10. Kohno M, Pouyssegur J. Targeting the ERK signaling pathway in cancer therapy. Ann Med. 2006. 38:200–211.
Article11. Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006. 24:21–44.
Article12. Nakayama N, Nakayama K, Yeasmin S, Ishibashi M, Katagiri A, Iida K, et al. KRAS or BRAF mutation status is a useful predictor of sensitivity to MEK inhibition in ovarian cancer. Br J Cancer. 2008. 99:2020–2028.
Article13. Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, et al. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004. 202:336–340.14. Cho YH, Kim DY, Kim JH, Kim YM, Kim KR, Nam JH, et al. Mutational analysis of KRAS, BRAF, and TP53 genes of ovarian serous carcinomas in Korean women. Yonsei Med J. 2009. 50:266–272.
Article15. Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature. 2006. 439:358–362.
Article16. Mayo MW, Norris JL, Baldwin AS. Ras regulation of NF-kappa B and apoptosis. Methods Enzymol. 2001. 333:73–87.17. Tran SE, Holmstrom TH, Ahonen M, Kahari VM, Eriksson JE. MAPK/ERK overrides the apoptotic signaling from Fas, TNF, and TRAIL receptors. J Biol Chem. 2001. 276:16484–16490.
Article18. Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006. 66:3859–3868.
Article19. Dent P, Grant S. Pharmacologic interruption of the mitogen-activated extracellular-regulated kinase/mitogen-activated protein kinase signal transduction pathway: potential role in promoting cytotoxic drug action. Clin Cancer Res. 2001. 7:775–783.20. Davies BR, Logie A, McKay JS, Martin P, Steele S, Jenkins R, et al. AZD6244 (ARRY-142886), a potent inhibitor of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase 1/2 kinases: mechanism of action in vivo, pharmacokinetic/pharmacodynamic relationship, and potential for combination in preclinical models. Mol Cancer Ther. 2007. 6:2209–2219.
Article21. MacKeigan JP, Collins TS, Ting JP. MEK inhibition enhances paclitaxel-induced tumor apoptosis. J Biol Chem. 2000. 275:38953–38956.
Article22. Leboeuf R, Baumgartner JE, Benezra M, Malaguarnera R, Solit D, Pratilas CA, et al. BRAFV600E mutation is associated with preferential sensitivity to mitogen-activated protein kinase kinase inhibition in thyroid cancer cell lines. J Clin Endocrinol Metab. 2008. 93:2194–2201.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Targeting BRAF pathway in low-grade serous ovarian cancer
- SP1-induced lncRNA MCF2L-AS1 promotes cisplatin resistance in ovarian cancer by regulating IGF2BP1/IGF2/MEK/ERK axis
- Apoptotic effect of NV-196, an isofl avone derivative, in epithelial ovarian cancer cells
- Expression of p21WAF1/CIP1/SDI1 in Epithelial Ovarian Cancer ; Its Relationship with p53 Expression and Prognostic Factors
- Ellagic Acid Shows Different Anti-proliferative Effects Between the MDA-MB-231 and MCF-7 Human Breast Cancer Cell Lines