Korean J Nutr.
2011 Oct;44(5):355-365. 10.4163/kjn.2011.44.5.355.
Effects of Different Types of Dietary Fat on Muscle Atrophy According to Muscle Fiber Types and PPARdelta Expression in Hindlimb-Immobilized Rats
- Affiliations
-
- 1Department of Food and Nutrition/Research Institute of Human Ecology Seoul National University, Seoul 151-742, Korea. lysook@snu.ac.kr
- KMID: 2268559
- DOI: http://doi.org/10.4163/kjn.2011.44.5.355
Abstract
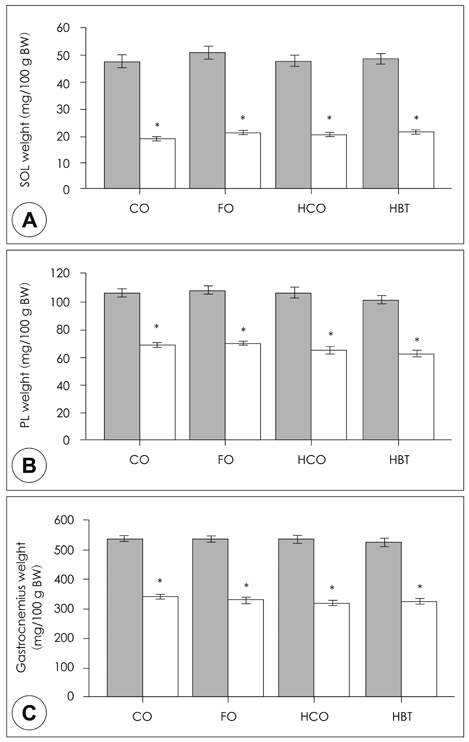
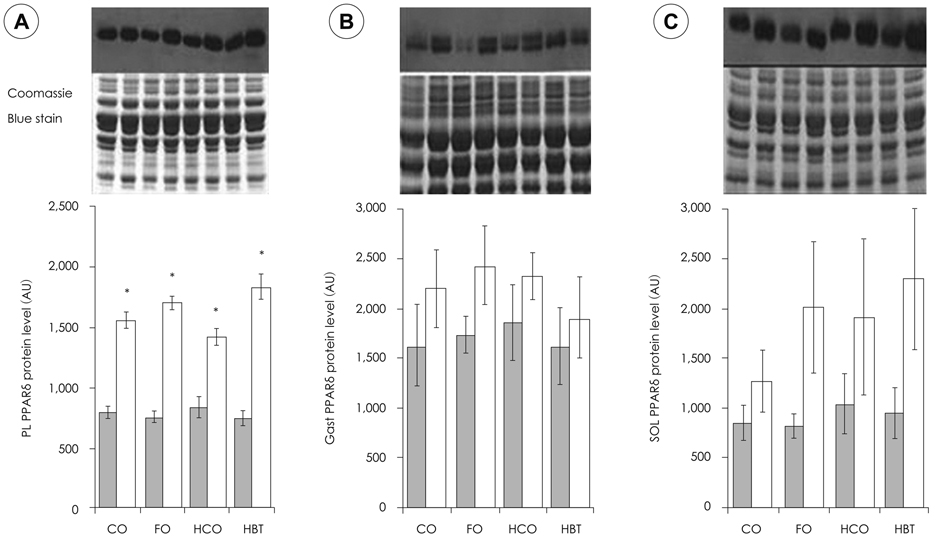
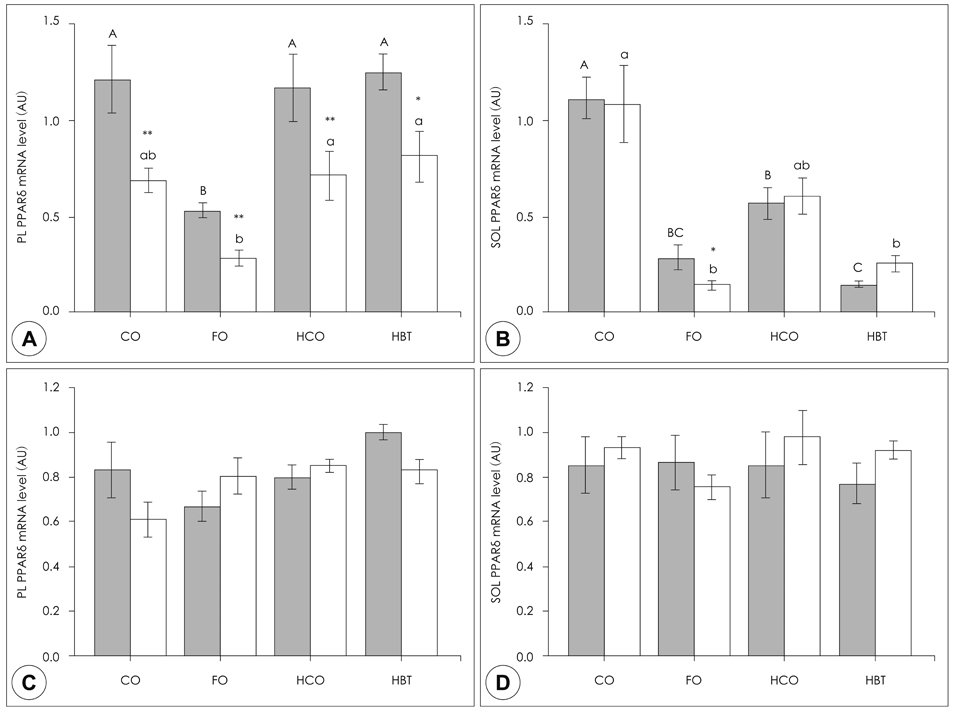
- This study investigated how dietary fat affects muscle atrophy and lipid metabolism in various muscles during hindlimb immobilization in rats. Twenty-four male Sprague-Dawley rats had their left hindlimb immobilized and were divided into four groups by dietary fat content and composition. The contralateral hindlimb (control) was compared with the immobilized limb in all dietary groups. Rats (n = 6/group) were fed a 4% corn oil diet (CO), 2.6% corn oil + 1.4% fish oil diet (FO), 30% corn oil diet (HCO), or a 30% beef tallow diet (HBT)after their hind limbs were immobilized for 10 days. Data were collected for the gastrocnemius, plantaris and soleus muscles. Muscle atrophy was induced significantly after 10 days of hindlimb immobilization, resulting in significantly decreased muscle mass and total muscle protein content. The protein levels of peroxisome proliferator activated receptor delta (PPARdelta) in the plantaris, gastrocnemius, and soleus increased following hindlimb immobilization irrespective of dietary fat intake. Interestingly, the PPARdelta mRNA level in the plantaris decreased significantly in all groups and that in the FO group was lower than that in the other groups. The soleus PPARdelta mRNA level decreased significantly following hindlimb immobilization in the FO group only. Muscle carnitine palmitoyl transferase 1 (mCPT1) mRNA level was not affected by hindlimb immobilization. However, the mCPT1 mRNA level in the FO group was significantly lower in the plantaris but higher in the soleus than that in the other groups. The pyruvate dehydrogenase kinase 4 (PDK4) mRNA level in the plantaris decreased significantly, whereas that in the soleus increased significantly following hindlimb immobilization. The plantaris, but not soleus, PDK4 mRNA level was significantly higher in the FO group than that in the CO group. The increased PPARdelta protein level following hindlimb immobilization may have suppressed triglyceride accumulation in muscles and different types of dietary fat may have differentially affected muscle atrophy according to muscle type. Our results suggest that omega-3 polyunsaturated fatty acids may suppress muscle atrophy and lipid accumulation by positively affecting the expression level and activity of PPARdelta and PPARdelta-related enzymes, which are supposed to play an important role in muscle lipid metabolism.
MeSH Terms
-
Animals
Carnitine
Corn Oil
Diet
Diet, High-Fat
Dietary Fats
Extremities
Fats
Fatty Acids, Unsaturated
Hindlimb
Hindlimb Suspension
Humans
Lipid Metabolism
Male
Muscle Proteins
Muscles
Muscular Atrophy
Oxidoreductases
Phosphotransferases
PPAR delta
Protein Kinases
Pyruvic Acid
Rats
Rats, Sprague-Dawley
RNA, Messenger
Transferases
Carnitine
Corn Oil
Dietary Fats
Fats
Fatty Acids, Unsaturated
Muscle Proteins
Oxidoreductases
PPAR delta
Phosphotransferases
Protein Kinases
Pyruvic Acid
RNA, Messenger
Transferases
Figure
Reference
-
1. You JS, Park MN, Song W, Lee YS. Dietary fish oil alleviates soleus atrophy during immobilization in association with Akt signaling to p70s6k and E3 ubiquitin ligases in rats. Appl Physiol Nutr Metab. 2010. 35(3):310–318.
Article2. You JS, Park MN, Lee YS. Dietary fish oil inhibits the early stage of recovery of atrophied soleus muscle in rats via Akt-p70s6k signaling and PGF2α. J Nutr Biochem. 2010. 21(10):929–934.
Article3. Lindboe CF, Platou CS. Effect of immobilization of short duration on the muscle fibre size. Clin Physiol. 1984. 4(2):183–188.
Article4. Fitts RH, Riley DR, Widrick JJ. Functional and structural adaptations of skeletal muscle to microgravity. J Exp Biol. 2001. 204(Pt 18):3201–3208.
Article5. Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004. 2(10):e294.6. Montell E, Turini M, Marotta M, Roberts M, Noé V, Ciudad CJ, Macé K, Gómez-Foix AM. DAG accumulation from saturated fatty acids desensitizes insulin stimulation of glucose uptake in muscle cells. Am J Physiol Endocrinol Metab. 2001. 280(2):E229–E237.
Article7. Ehrenborg E, Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol Rev. 2009. 61(3):373–393.
Article8. Mazzatti DJ, Smith MA, Oita RC, Lim FL, White AJ, Reid MB. Muscle unloading-induced metabolic remodeling is associated with acute alterations in PPARdelta and UCP-3 expression. Physiol Genomics. 2008. 34(2):149–161.
Article9. Grimaldi PA. Regulatory role of peroxisome proliferator-activated receptor delta (PPAR delta) in muscle metabolism. A new target for metabolic syndrome treatment? Biochimie. 2005. 87(1):5–8.
Article10. Sinha S, Perdomo G, Brown NF, O'Doherty RM. Fatty acid-induced insulin resistance in L6 myotubes is prevented by inhibition of activation and nuclear localization of nuclear factor kappa B. J Biol Chem. 2004. 279(40):41294–41301.
Article11. Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001. 98(4):2005–2010.
Article12. Mullen KL, Pritchard J, Ritchie I, Snook LA, Chabowski A, Bonen A, Wright D, Dyck DJ. Adiponectin resistance precedes the accumulation of skeletal muscle lipids and insulin resistance in high-fat-fed rats. Am J Physiol Regul Integr Comp Physiol. 2009. 296(2):R243–R251.
Article13. Marcó A, Rubio R, Compañó R, Casals I. Comparison of the Kjeldahl method and a combustion method for total nitrogen determination in animal feed. Talanta. 2002. 57(5):1019–1026.
Article14. Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957. 226(1):497–509.
Article15. Vazeille E, Codran A, Claustre A, Averous J, Listrat A, Béchet D, Taillandier D, Dardevet D, Attaix D, Combaret L. The ubiquitin-proteasome and the mitochondria-associated apoptotic pathways are sequentially downregulated during recovery after immobilization-induced muscle atrophy. Am J Physiol Endocrinol Metab. 2008. 295(5):E1181–E1190.
Article16. Widrick JJ, Maddalozzo GF, Hu H, Herron JC, Iwaniec UT, Turner RT. Detrimental effects of reloading recovery on force, shortening velocity, and power of soleus muscles from hindlimb-unloaded rats. Am J Physiol Regul Integr Comp Physiol. 2008. 295(5):R1585–R1592.
Article17. Krawiec BJ, Frost RA, Vary TC, Jefferson LS, Lang CH. Hindlimb casting decreases muscle mass in part by proteasome-dependent proteolysis but independent of protein synthesis. Am J Physiol Endocrinol Metab. 2005. 289(6):E969–E980.
Article18. Bajotto G, Sato Y, Kitaura Y, Shimomura Y. Effect of branched-chain amino acid supplementation during unloading on regulatory components of protein synthesis in atrophied soleus muscles. Eur J Appl Physiol. 2011. 111(8):1815–1828.
Article19. Gustafsson T, Osterlund T, Flanagan JN, von Waldén F, Trappe TA, Linnehan RM, Tesch PA. Effects of 3 days unloading on molecular regulators of muscle size in humans. J Appl Physiol. 2010. 109(3):721–727.
Article20. Krämer DK, Ahlsén M, Norrbom J, Jansson E, Hjeltnes N, Gustafsson T, Krook A. Human skeletal muscle fibre type variations correlate with PPAR alpha, PPAR delta and PGC-1 alpha mRNA. Acta Physiol (Oxf). 2006. 188(3-4):207–216.
Article21. Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005. 19(8):986–988.
Article22. Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A. 1997. 94(9):4312–4317.
Article23. Hancock CR, Han DH, Chen M, Terada S, Yasuda T, Wright DC, Holloszy JO. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Proc Natl Acad Sci U S A. 2008. 105(22):7815–7820.
Article24. Stein T, Schluter M, Galante A, Soteropoulos P, Tolias P, Grindeland R, Moran M, Wang T, Polansky M, Wade C. Energy metabolism pathways in rat muscle under conditions of simulated microgravity. J Nutr Biochem. 2002. 13(8):471.
Article25. Nahlé Z, Hsieh M, Pietka T, Coburn CT, Grimaldi PA, Zhang MQ, Das D, Abumrad NA. CD36-dependent regulation of muscle FoxO1 and PDK4 in the PPARdelta/beta-mediated adaptation to metabolic stress. J Biol Chem. 2008. 283(21):14317–14326.
Article26. Power GW, Newsholme EA. Dietary fatty acids influence the activity and metabolic control of mitochondrial carnitine palmitoyltransferase I in rat heart and skeletal muscle. J Nutr. 1997. 127(11):2142–2150.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Periodic Walking on the Type II Muscle of Growing Suspended Rats
- Effect of Articular Immobilization - Induced Hindlimb Skeletal Muscle Atrophy on Autophosphorylation of Tyrosine Kinase of the Insulin Receptor in Rats
- Effect of Endurance Exercise Prior to Occurrence of Muscle Atrophy on the Mass, Myofibrillar Protein Content and Fiber Crossectional Area of Atrophied Hindlimb Muscles of Rats
- Experimental Studies on Disuse Atrophy of the Rat Tibialis Anterior Muscle
- Effects of Unilateral Sciatic Nerve Injury on Unaffected Hindlimb Muscles of Rats