Ann Dermatol.
2014 Dec;26(6):713-721. 10.5021/ad.2014.26.6.713.
Expression Patterns of Gli-1, Pleckstrin Homology-Like Domain, Family A, Member 1, Transforming Growth Factor-beta1/beta2, and p63 in Sebaceous and Follicular Tumors
- Affiliations
-
- 1Department of Dermatology, Dong-A University College of Medicine, Busan, Korea. khkim@dau.ac.kr
- 2Department of Pathology, Dong-A University College of Medicine, Busan, Korea.
- KMID: 2264866
- DOI: http://doi.org/10.5021/ad.2014.26.6.713
Abstract
- BACKGROUND
Certain epidermal appendage tumors, including hyperplasias (hamartomas), adenomas, benign epitheliomas, primordial epitheliomas, and malignant tumors, can exhibit any stage of differentiation. Several molecules associated with tumorigenesis, such as Gli-1, pleckstrin homology-like domain, family A, member 1 (PHLDA-1), transforming growth factor (TGF)-beta1, TGF-beta2, and p63, are associated with tumor grade and aggressive behavior in follicular and sebaceous tumors in ways that are not well understood.
OBJECTIVE
The aim of this study was to elucidate the expression of Gli-1, PHLDA-1, TGF-beta1/beta2, and p63 in benign and malignant tumors of the hair and sebaceous glands and to determine their importance in the degree of tumor differentiation.
METHODS
Immunohistochemistry was performed in follicular and sebaceous tumors using antibodies against Gli-1 (sebaceous tumor marker), PHLDA-1 (hair follicle outer root sheath [ORS] cell marker), p63, TGF-beta1, and TGF-beta2.
RESULTS
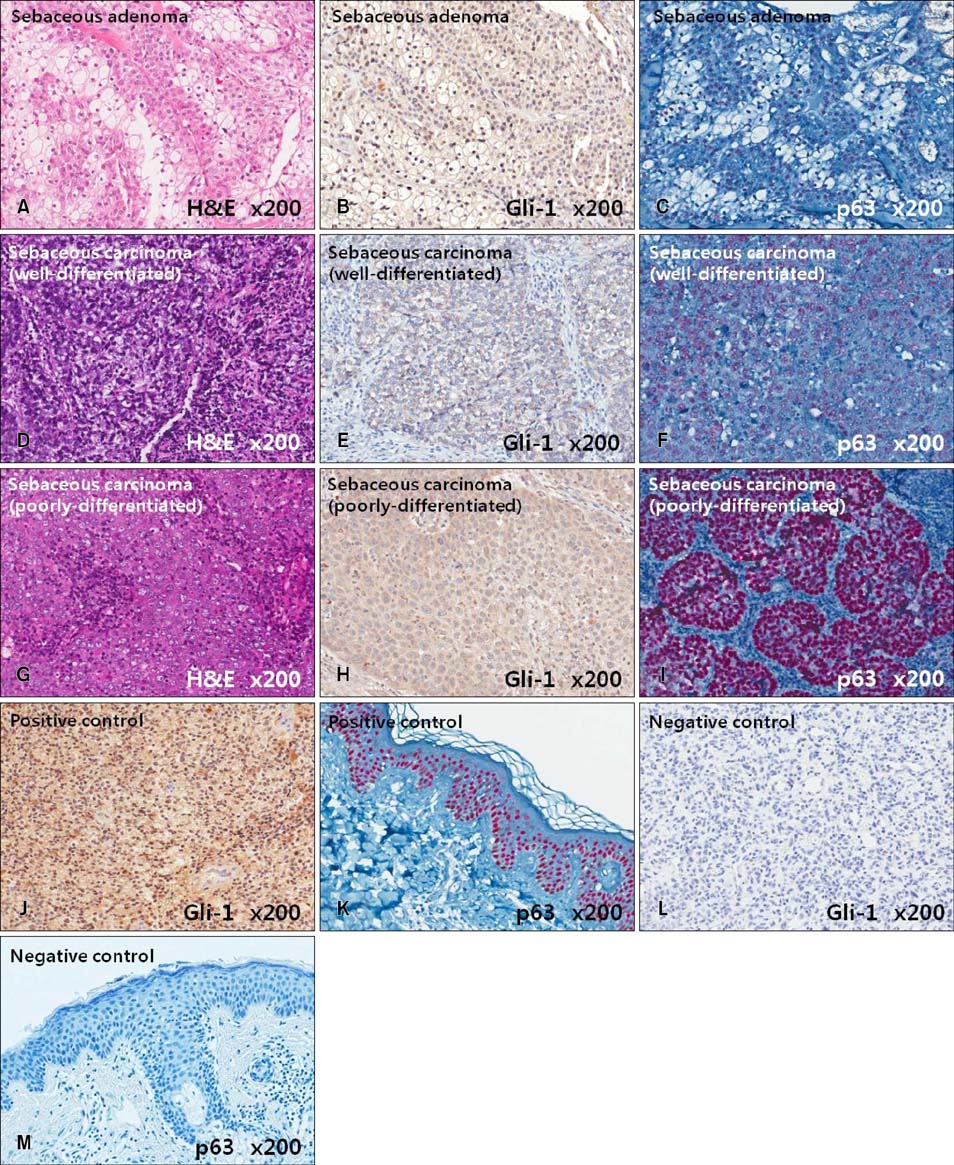
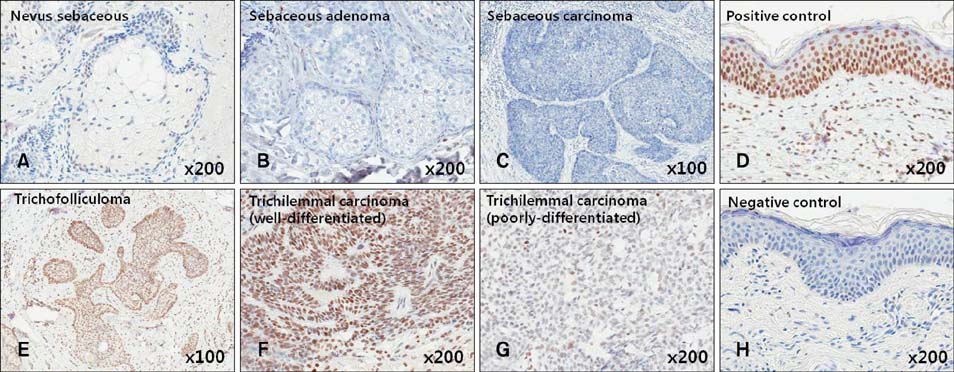
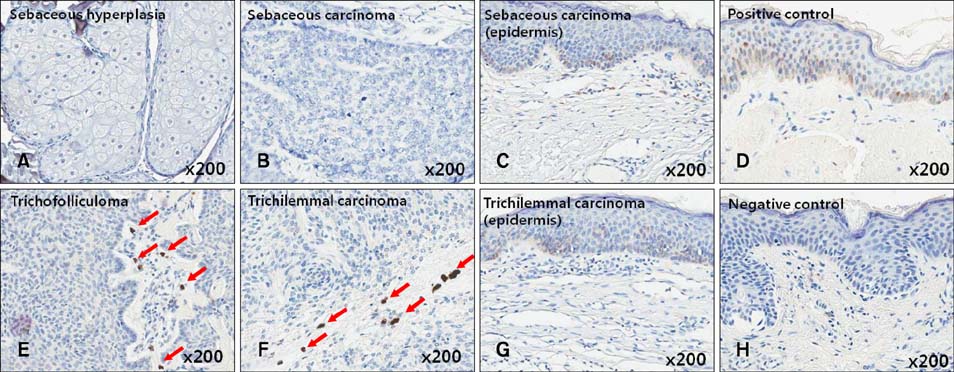
Gli-1 was expressed in basaloid cells, sebocytes, and sebaceous carcinoma cells, and expression levels decreased as differentiation progressed. PHLDA-1 was expressed in ORS cells and some follicular tumor cells. Expression of p63 was observed in the nuclei of the outermost basaloid cells (seboblasts), poorly differentiated sebaceous carcinoma cells, and tumor cells toward the direction of the hair. Remarkably, TGF-beta1 was expressed exclusively in the nuclei of benign and malignant follicular (hair) tumors, but not in sebaceous tumors, at levels that correlated with the degree of differentiation.
CONCLUSION
We propose that p63 and/or TGF-beta1 are useful for predicting the degree of differentiation and malignant potential of sebaceous and follicular tumors and for distinguishing trichilemmal carcinoma from sebaceous carcinoma.
MeSH Terms
Figure
Reference
-
1. Panteleyev AA, Jahoda CA, Christiano AM. Hair follicle predetermination. J Cell Sci. 2001; 114:3419–3431.
Article2. Niemann C, Watt FM. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 2002; 12:185–192.
Article3. Fuchs E, Raghavan S. Getting under the skin of epidermal morphogenesis. Nat Rev Genet. 2002; 3:199–209.
Article4. Taylor G, Lehrer MS, Jensen PJ, Sun TT, Lavker RM. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell. 2000; 102:451–461.
Article5. Turusov VS, Mohr U. Pathology of tumours in laboratory animals. Volume II, tumours of the mouse. Lyon, France: International Agency for Research on Cancer;1994. p. 1–26.6. Villavicencio EH, Walterhouse DO, Iannaccone PM. The sonic hedgehog-patched-gli pathway in human development and disease. Am J Hum Genet. 2000; 67:1047–1054.
Article7. Niemann C, Unden AB, Lyle S, Zouboulis ChC, Toftgård R, Watt FM. Indian hedgehog and beta-catenin signaling: role in the sebaceous lineage of normal and neoplastic mammalian epidermis. Proc Natl Acad Sci U S A. 2003; 100:Suppl 1. 11873–11880.
Article8. Ohyama M, Terunuma A, Tock CL, Radonovich MF, Pise-Masison CA, Hopping SB, et al. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J Clin Invest. 2006; 116:249–260.
Article9. Sellheyer K, Krahl D. PHLDA1 (TDAG51) is a follicular stem cell marker and differentiates between morphoeic basal cell carcinoma and desmoplastic trichoepithelioma. Br J Dermatol. 2011; 164:141–147.
Article10. Heldin CH, Landström M, Moustakas A. Mechanism of TGF-beta signaling to growth arrest, apoptosis, and epithelialmesenchymal transition. Curr Opin Cell Biol. 2009; 21:166–176.
Article11. Wakefield LM, Roberts AB. TGF-beta signaling: positive and negative effects on tumorigenesis. Curr Opin Genet Dev. 2002; 12:22–29.12. Stecca B, Ruiz i Altaba A. A GLI1-p53 inhibitory loop controls neural stem cell and tumour cell numbers. EMBO J. 2009; 28:663–676.
Article13. Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007; 17:165–172.
Article14. Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am J Pathol. 2007; 170:1445–1453.
Article15. Viganò MA, Mantovani R. Hitting the numbers: the emerging network of p63 targets. Cell Cycle. 2007; 6:233–239.
Article16. Perez CA, Pietenpol JA. Transcriptional programs regulated by p63 in normal epithelium and tumors. Cell Cycle. 2007; 6:246–254.
Article17. Lo Muzio L, Santarelli A, Caltabiano R, Rubini C, Pieramici T, Trevisiol L, et al. p63 overexpression associates with poor prognosis in head and neck squamous cell carcinoma. Hum Pathol. 2005; 36:187–194.
Article18. Gu X, Coates PJ, Boldrup L, Nylander K. p63 contributes to cell invasion and migration in squamous cell carcinoma of the head and neck. Cancer Lett. 2008; 263:26–34.
Article19. Tsujita-Kyutoku M, Kiuchi K, Danbara N, Yuri T, Senzaki H, Tsubura A. p63 expression in normal human epidermis and epidermal appendages and their tumors. J Cutan Pathol. 2003; 30:11–17.
Article20. Qureshi HS, Ormsby AH, Lee MW, Zarbo RJ, Ma CK. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004; 31:145–152.
Article21. Plaza JA, Ortega PF, Stockman DL, Suster S. Value of p63 and podoplanin (D2-40) immunoreactivity in the distinction between primary cutaneous tumors and adenocarcinomas metastatic to the skin: a clinicopathologic and immunohistochemical study of 79 cases. J Cutan Pathol. 2010; 37:403–410.
Article22. Mahalingam M, Nguyen LP, Richards JE, Muzikansky A, Hoang MP. The diagnostic utility of immunohistochemistry in distinguishing primary skin adnexal carcinomas from metastatic adenocarcinoma to skin: an immunohistochemical reappraisal using cytokeratin 15, nestin, p63, D2-40, and calretinin. Mod Pathol. 2010; 23:713–719.
Article23. Akhurst RJ, Derynck R. TGF-beta signaling in cancer--a double-edged sword. Trends Cell Biol. 2001; 11:S44–S51.24. Katsuno Y, Lamouille S, Derynck R. TGF-β signaling and epithelial-mesenchymal transition in cancer progression. Curr Opin Oncol. 2013; 25:76–84.
Article25. Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, et al. A Mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009; 137:87–98.
Article26. Soma T, Tsuji Y, Hibino T. Involvement of transforming growth factor-beta2 in catagen induction during the human hair cycle. J Invest Dermatol. 2002; 118:993–997.
Article27. Inoue K, Aoi N, Yamauchi Y, Sato T, Suga H, Eto H, et al. TGF-beta is specifically expressed in human dermal papilla cells and modulates hair folliculogenesis. J Cell Mol Med. 2009; 13:4643–4656.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Transforming Growth Factor -beta1, -beta2 in Human Endometrium of The Uterine Adenocarcinoma
- Expression of TGF-beta isoforms in the Human Fetal Hepatic Erythropoiesis
- Expression of Transforming Growth Factor-beta1 , beta2 by Immunohistochemical Staining method: In Human Endometrium through the Menstrual Cycle
- Differential Expression of TGF-beta Isoforms in Human Kerationocytes by Narrow Band UVB
- Prognostic Value of TGF-beta1, TGF-beta2 Expression and Ki-67 Labelling Index in Prostate Cancer