Anat Cell Biol.
2013 Sep;46(3):191-197. 10.5115/acb.2013.46.3.191.
Stereological study of the effects of morphine consumption and abstinence on the number of the neurons and oligodendrocytes in medial prefrontal cortex of rats
- Affiliations
-
- 1Histomorphometry and Stereology Research Center, Shiraz University of Medical Sciences, Shiraz, Iran. noora@sums.ac.ir
- 2Department of Physiology, Shiraz University of Medical Sciences, Shiraz, Iran.
- KMID: 2263145
- DOI: http://doi.org/10.5115/acb.2013.46.3.191
Abstract
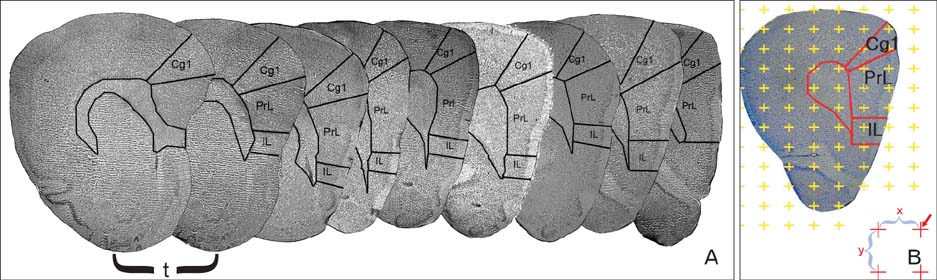
- Quantitative studies to date on the effects of opioid consumption and abstinence on the nervous system using modern stereological methods have not received enough attention. In addition, they have yielded controversial results. The present study was conducted to investigate the effects of morphine, with or without abstinence, on the neurons and oligodendrocytes of the medial prefrontal cortex (MPFC) in rats using quantitative stereological methods. The male rats were divided into four groups: the first (saline [SAL]) and second (morphine [MOR]) groups were treated with saline and an escalating dose of morphine (5-20 mg/kg) for 30 days, respectively; the third (SAL+abstinence [ABS]) and fourth (MOR+ABS) groups were treated in the same manner as the previous groups plus they had a 30-day abstinence period. The results showed that the volume of the MPFC and its subdivisions decreased by approximately 15% in the MOR group compared with that in the SAL group (P<0.05). In addition, the volume decreased by approximately 24% in the MOR+ABS group compared with that in the SAL+ABS group (P<0.05). The number of neurons in the MOR and MOR+ABS groups decreased by approximately 44% and 35%, respectively, compared with that in their corresponding control groups. Moreover, the number of the oligodendrocytes in the MOR and MOR+ABS groups decreased by approximately 41% and 37%, respectively. No significant difference was noted in the number of cells in the MOR and MOR+ABS groups. In conclusion, morphine consumption leads to a permanent reduction in the number of neurons and oligodendrocytes, and no additional neuron and oligodendrocyte loss occurs after abstinence.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats
Adekomi Damilare Adedayo, Adegoke Adebiyi Aderinola, Tijani Ahmad Adekilekun, Olaniyan Olayinka Olaolu, Alabi Mutiyat Olanike, Ijomone Kafilat Olayemi
Anat Cell Biol. 2018;51(1):41-51. doi: 10.5115/acb.2018.51.1.41.
Reference
-
1. Liu LW, Lu J, Wang XH, Fu SK, Li Q, Lin FQ. Neuronal apoptosis in morphine addiction and its molecular mechanism. Int J Clin Exp Med. 2013; 6:540–545.2. Spiga S, Puddu MC, Pisano M, Diana M. Morphine withdrawal-induced morphological changes in the nucleus accumbens. Eur J Neurosci. 2005; 22:2332–2340.3. Fattore L, Puddu MC, Picciau S, Cappai A, Fratta W, Serra GP, Spiga S. Astroglial in vivo response to cocaine in mouse dentate gyrus: a quantitative and qualitative analysis by confocal microscopy. Neuroscience. 2002; 110:1–6.4. Ballesteros-Yáñez I, Ambrosio E, Benavides-Piccione R, Pérez J, Torres I, Miguéns M, García-Lecumberri C, DeFelipe J. The effects of morphine self-administration on cortical pyramidal cell structure in addiction-prone lewis rats. Cereb Cortex. 2007; 17:238–249.5. Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008; 32:386–394.6. Pezawas LM, Fischer G, Diamant K, Schneider C, Schindler SD, Thurnher M, Ploechl W, Eder H, Kasper S. Cerebral CT findings in male opioid-dependent patients: stereological, planimetric and linear measurements. Psychiatry Res. 1998; 83:139–147.7. Amass L, Nardin R, Mendelson JH, Teoh SK, Woods BT. Quantitative magnetic resonance imaging in heroin- and cocaine-dependent men: a preliminary study. Psychiatry Res. 1992; 45:15–23.8. Lyoo IK, Pollack MH, Silveri MM, Ahn KH, Diaz CI, Hwang J, Kim SJ, Yurgelun-Todd DA, Kaufman MJ, Renshaw PF. Prefrontal and temporal gray matter density decreases in opiate dependence. Psychopharmacology (Berl). 2006; 184:139–144.9. Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ. Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. Am J Psychiatry. 2001; 158:1680–1686.10. Rapeli P, Kivisaari R, Autti T, Kähkönen S, Puuskari V, Jokela O, Kalska H. Cognitive function during early abstinence from opioid dependence: a comparison to age, gender, and verbal intelligence matched controls. BMC Psychiatry. 2006; 6:9.11. Mann K, Agartz I, Harper C, Shoaf S, Rawlings RR, Momenan R, Hommer DW, Pfefferbaum A, Sullivan EV, Anton RF, Drobes DJ, George MS, Bares R, Machulla HJ, Mundle G, Reimold M, Heinz A. Neuroimaging in alcoholism: ethanol and brain damage. Alcohol Clin Exp Res. 2001; 25:5 Suppl ISBRA. 104S–109S.12. Zhang Y, Chen Q, Yu LC. Morphine: a protective or destructive role in neurons? Neuroscientist. 2008; 14:561–570.13. Tamura Y, Monden M, Shintani M, Kawai A, Shiomi H. Neuroprotective effects of hibernation-regulating substances against low-temperature-induced cell death in cultured hamster hippocampal neurons. Brain Res. 2006; 1108:107–116.14. Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002; 42:829–836.15. Emeterio EP, Tramullas M, Hurlé MA. Modulation of apoptosis in the mouse brain after morphine treatments and morphine withdrawal. J Neurosci Res. 2006; 83:1352–1361.16. Droblenkov AV, Karelina NR, Shabanov PD. Changes in neurons and gliocytes in the mesoaccumbocingulate system on perinatal exposure to morphine in rats. Neurosci Behav Physiol. 2010; 40:848–851.17. Nestler EJ. Molecular basis of long-term plasticity underlying addiction. Nat Rev Neurosci. 2001; 2:119–128.18. Bonci A, Williams JT. Increased probability of GABA release during withdrawal from morphine. J Neurosci. 1997; 17:796–803.19. Manzoni OJ, Williams JT. Presynaptic regulation of glutamate release in the ventral tegmental area during morphine withdrawal. J Neurosci. 1999; 19:6629–6636.20. Van den Oever MC, Spijker S, Smit AB, De Vries TJ. Prefrontal cortex plasticity mechanisms in drug seeking and relapse. Neurosci Biobehav Rev. 2010; 35:276–284.21. Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012; 17:132–141.22. Kristiansen SL, Nyengaard JR. Digital stereology in neuropathology. APMIS. 2012; 120:327–340.23. Hyde DM, Tyler NK, Plopper CG. Morphometry of the respiratory tract: avoiding the sampling, size, orientation, and reference traps. Toxicol Pathol. 2007; 35:41–48.24. Li Q, Zhao X, Zhong LJ, Yang HY, Wang Q, Pu XP. Effects of chronic morphine treatment on protein expression in rat dorsal root ganglia. Eur J Pharmacol. 2009; 612:21–28.25. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. San Diego: Elsevier Academic Press;2005.26. Davanlou M, Smith DF. Unbiased stereological estimation of different cell types in rat cerebral cortex. Image Anal Stereol. 2004; 23:1–11.27. Chareyron LJ, Lavenex PB, Lavenex P. Postnatal development of the amygdala: a stereological study in rats. J Comp Neurol. 2012; 520:3745–3763.28. Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988; 96:379–394.29. Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Møller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988; 96:857–881.30. Boyce RW, Dorph-Petersen KA, Lyck L, Gundersen HJ. Design-based stereology: introduction to basic concepts and practical approaches for estimation of cell number. Toxicol Pathol. 2010; 38:1011–1025.31. Hodgson PS, Neal JM, Pollock JE, Liu SS. The neurotoxicity of drugs given intrathecally (spinal). Anesth Analg. 1999; 88:797–809.32. Mao J, Sung B, Ji RR, Lim G. Neuronal apoptosis associated with morphine tolerance: evidence for an opioid-induced neurotoxic mechanism. J Neurosci. 2002; 22:7650–7661.33. Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci. 2004; 114:1001–1011.34. Turchan-Cholewo J, Liu Y, Gartner S, Reid R, Jie C, Peng X, Chen KC, Chauhan A, Haughey N, Cutler R, Mattson MP, Pardo C, Conant K, Sacktor N, McArthur JC, Hauser KF, Gairola C, Nath A. Increased vulnerability of ApoE4 neurons to HIV proteins and opiates: protection by diosgenin and L-deprenyl. Neurobiol Dis. 2006; 23:109–119.35. Boronat MA, García-Fuster MJ, García-Sevilla JA. Chronic morphine induces up-regulation of the pro-apoptotic Fas receptor and down-regulation of the anti-apoptotic Bcl-2 oncoprotein in rat brain. Br J Pharmacol. 2001; 134:1263–1270.36. Peart JN, Gross ER, Gross GJ. Opioid-induced preconditioning: recent advances and future perspectives. Vascul Pharmacol. 2005; 42:211–218.37. Rambhia S, Mantione KJ, Stefano GB, Cadet P. Morphine modulation of the ubiquitin-proteasome complex is neuroprotective. Med Sci Monit. 2005; 11:BR386–BR396.38. Zhang W, Hong JS, Kim HC, Zhang W, Block ML. Morphinan neuroprotection: new insight into the therapy of neurodegeneration. Crit Rev Neurobiol. 2004; 16:271–302.39. Bekheet SH, Saker SA, Abdel-Kader AM, Younis AE. Histopathological and biochemical changes of morphine sulphate administration on the cerebellum of albino rats. Tissue Cell. 2010; 42:165–175.40. Spiga S, Serra GP, Puddu MC, Foddai M, Diana M. Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur J Neurosci. 2003; 17:605–612.41. Zehr C, Lewis J, McGowan E, Crook J, Lin WL, Godwin K, Knight J, Dickson DW, Hutton M. Apoptosis in oligodendrocytes is associated with axonal degeneration in P301L tau mice. Neurobiol Dis. 2004; 15:553–562.42. Namavar MR, Raminfard S, Jahromi ZV, Azari H. Effects of high-fat diet on the numerical density and number of neuronal cells and the volume of the mouse hypothalamus: a stereological study. Anat Cell Biol. 2012; 45:178–184.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Changes in Neural Activity of Prefrontal Cortical Neurons of Behaving Rats by Clozapine Administration
- Morphine-alcohol treatment impairs cognitive functions and increases neuro-inflammatory responses in the medial prefrontal cortex of juvenile male rats
- Role of Medial Prefrontal Cortical Neurons and Oxytocin Modulation in the Establishment of Social Buffering
- Stress Changes the Spatial Arrangement of Neurons and Glial Cells of Medial Prefrontal Cortex and Sertraline and Curcumin Prevent It
- An Autoradiographic Study on the Rat Neostriatal Dopamine Receptor Changes after 6-hydroxydopamine Injection into the Medial Prefrontal Cortex