Mechanisms of Asthma and Implications for Its Prevention and Treatment: A Personal Journey
- Affiliations
-
- 1Faculty of Medicine University of Southampton, UK. sth@soton.ac.uk
- KMID: 2260276
- DOI: http://doi.org/10.4168/aair.2013.5.6.343
Abstract
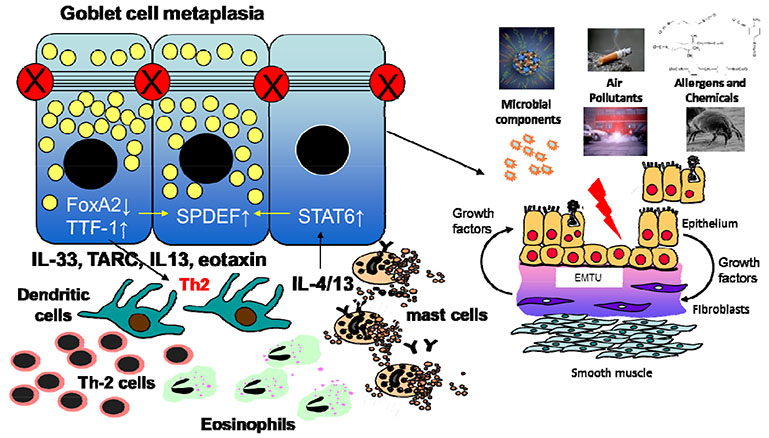
- My research career has focused on the causes of asthma and its treatment. After establishing the key role that mast cells play in the inflammatory response in asthma, attention was turned towards understanding disease chronicity and variability across the lifecourse. Through a combination of studies on airway biopsies and primary cell cultures we have established that asthma is primarily an epithelial disease driven by increased environmental susceptibility to injury and an altered repair response as depicted by sustained activation of the epithelial mesenchymal trophic unit (EMTU) that is invoked in foetal branching morphogenesis. Varied activation of the EMTU connects the origins of asthma to its progression over time with involvement of epithelial susceptibility through impaired barrier and innate immune functions and altered mesenchymal susceptibility as exemplified by polymorphisms of the metalloprotease gene, ADAM33. Taken together these observations have led to a fundamental re-evaluation of asthma pathogenesis. Rather than placing allergic inflammation as the prime abnormality, it is proposed that the airway epithelium lies at the center of asthma pathogenesis, and that in conjunction with the underlying mesenchyme, it is the principle orchestrator of both the induction of asthma and its evolution over the lifecourse. This concept has provided 'the basis for a new preventative and therapeutic approach focused more on increasing the airways resistance to environmental insults rather than suppressing downstream inflammation once it is established.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Particulate Matter 2.5 Causes Deficiency in Barrier Integrity in Human Nasal Epithelial Cells
Mu Xian, Siyuan Ma, Kuiji Wang, Hongfei Lou, Yang Wang, Luo Zhang, Chengshuo Wang, Cezmi A. Akdis
Allergy Asthma Immunol Res. 2020;12(1):56-71. doi: 10.4168/aair.2020.12.1.56.Association of TLR3 gene polymorphism with IgG subclass deficiency and the severity in patients with aspirin-intolerant asthma
Seung-Hyun Kim, Eun-Mi Yang, Hye-Min Jung, Duy Le Pham, Hyun-Na Choi, Ga-Young Ban, Hae-Sim Park
Allergy Asthma Respir Dis. 2016;4(4):264-270. doi: 10.4168/aard.2016.4.4.264.Airway epithelial cells in airway inflammation and remodeling in asthma
Sae-Hoon Kim
Allergy Asthma Respir Dis. 2016;4(2):82-90. doi: 10.4168/aard.2016.4.2.82.Role of inflammasome activation in development and exacerbation of asthma
Tae-Hyeong Lee, Hyun Ji Song, Choon-Sik Park
Asia Pac Allergy. 2014;4(4):187-196. doi: 10.5415/apallergy.2014.4.4.187.
Reference
-
1. Holgate ST, Baldwin CJ, Tattersfield AE. Beta-adrenergic agonist resistance in normal human airways. Lancet. 1977; 2:375–377.2. Howarth PH, Durham SR, Lee TH, Kay AB, Church MK, Holgate ST. Influence of albuterol, cromolyn sodium and ipratropium bromide on the airway and circulating mediator responses to allergen bronchial provocation in asthma. Am Rev Respir Dis. 1985; 132:986–992.3. Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol. 1983; 15:161–165.4. Bradding P, Feather IH, Howarth PH, Mueller R, Roberts JA, Britten K, Bews JP, Hunt TC, Okayama Y, Heusser CH, Bullock GR, Church MK, Holgate ST. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992; 176:1381–1386.5. Montefort S, Gratziou C, Goulding D, Polosa R, Haskard DO, Howarth PH, Holgate ST, Carroll MP. Bronchial biopsy evidence for leukocyte infiltration and upregulation of leukocyte-endothelial cell adhesion molecules 6 hours after local allergen challenge of sensitized asthmatic airways. J Clin Invest. 1994; 93:1411–1421.6. Beasley R, Roche WR, Roberts JA, Holgate ST. Cellular events in the bronchi in mild asthma and after bronchial provocation. Am Rev Respir Dis. 1989; 139:806–817.7. Djukanović R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, Howarth PH, Holgate ST. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. Am Rev Respir Dis. 1992; 145:669–674.8. Cowburn AS, Sladek K, Soja J, Adamek L, Nizankowska E, Szczeklik A, Lam BK, Penrose JF, Austen FK, Holgate ST, Sampson AP. Overexpression of leukotriene C4 synthase in bronchial biopsies from patients with aspirin-intolerant asthma. J Clin Invest. 1998; 101:834–846.9. Corne J, Djukanovic R, Thomas L, Warner J, Botta L, Grandordy B, Gygax D, Heusser C, Patalano F, Richardson W, Kilchherr E, Staehelin T, Davis F, Gordon W, Sun L, Liou R, Wang G, Chang TW, Holgate S. The effect of intravenous administration of a chimeric anti-IgE antibody on serum IgE levels in atopic subjects: efficacy, safety, and pharmacokinetics. J Clin Invest. 1997; 99:879–887.10. Roche WR, Beasley R, Williams JH, Holgate ST. Subepithelial fibrosis in the bronchi of asthmatics. Lancet. 1989; 1:520–524.11. Holgate ST, Davies DE, Lackie PM, Wilson SJ, Puddicombe SM, Lordan JL. Epithelial-mesenchymal interactions in the pathogenesis of asthma. J Allergy Clin Immunol. 2000; 105:193–204.12. Xiao C, Puddicombe SM, Field S, Haywood J, Broughton-Head V, Puxeddu I, Haitchi HM, Vernon-Wilson E, Sammut D, Bedke N, Cremin C, Sones J, Djukanović R, Howarth PH, Collins JE, Holgate ST, Monk P, Davies DE. Defective epithelial barrier function in asthma. J Allergy Clin Immunol. 2011; 128:549–556.e1-12.13. Maeda Y, Chen G, Xu Y, Haitchi HM, Du L, Keiser AR, Howarth PH, Davies DE, Holgate ST, Whitsett JA. Airway epithelial transcription factor NK2 homeobox 1 inhibits mucous cell metaplasia and Th2 inflammation. Am J Respir Crit Care Med. 2011; 184:421–429.14. Grainge CL, Lau LC, Ward JA, Dulay V, Lahiff G, Wilson S, Holgate S, Davies DE, Howarth PH. Effect of bronchoconstriction on airway remodeling in asthma. N Engl J Med. 2011; 364:2006–2015.15. Van Eerdewegh P, Little RD, Dupuis J, Del Mastro RG, Falls K, Simon J, Torrey D, Pandit S, McKenny J, Braunschweiger K, Walsh A, Liu Z, Hayward B, Folz C, Manning SP, Bawa A, Saracino L, Thackston M, Benchekroun Y, Capparell N, Wang M, Adair R, Feng Y, Dubois J, FitzGerald MG, Huang H, Gibson R, Allen KM, Pedan A, Danzig MR, Umland SP, Egan RW, Cuss FM, Rorke S, Clough JB, Holloway JW, Holgate ST, Keith TP. Association of the ADAM33 gene with asthma and bronchial hyperresponsiveness. Nature. 2002; 418:426–430.16. Johnston SL, Pattemore PK, Sanderson G, Smith S, Lampe F, Josephs L, Symington P, O'Toole S, Myint SH, Tyrrell DA, Holgate ST. Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ. 1995; 310:1225–1229.17. Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002; 359:831–834.18. Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995; 151:879–886.19. Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005; 201:937–947.20. Bjornsdottir US, Holgate ST, Reddy PS, Hill AA, McKee CM, Csimma CI, Weaver AA, Legault HM, Small CG, Ramsey RC, Ellis DK, Burke CM, Thompson PJ, Howarth PH, Wardlaw AJ, Bardin PG, Bernstein DI, Irving LB, Chupp GL, Bensch GW, Bensch GW, Stahlman JE, Karetzky M, Baker JW, Miller RL, Goodman BH, Raible DG, Goldman SJ, Miller DK, Ryan JL, Dorner AJ, Immermann FW, O'Toole M. Pathways activated during human asthma exacerbation as revealed by gene expression patterns in blood. PLoS One. 2011; 6:e21902.21. Holgate ST. The sentinel role of the airway epithelium in asthma pathogenesis. Immunol Rev. 2011; 242:205–219.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Embarking on a New Journey With the Allergy, Asthma & Immunology Research
- Influence of Asthma Epidemiology on the Risk for Other Diseases
- Allergic rhinitis, sinusitis and asthma: evidence for respiratory system integration
- Development of a PDA based Personal Asthma Management System
- The Clinical Impacts of Asthma to Infectious Diseases