Allergy Asthma Immunol Res.
2014 Nov;6(6):478-484. 10.4168/aair.2014.6.6.478.
The Effects of Environmental Toxins on Allergic Inflammation
- Affiliations
-
- 1Department of Pediatrics, E-DA Hospital, Kaohsiung, Taiwan.
- 2School of Medicine, College of Medicine, I-Shou University, Kaohsiung, Taiwan.
- 3Division of Cardiac Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 4Division of Cardiology, Department of Internal Medicine, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 5Department of Pediatrics, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan. kuochanghung.dr@gmail.com
- 6Department of Pediatrics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan. pedhung@gmail.com
- 7Department of Radiation Oncology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
- 8Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
- 9Department of Pediatrics, Kaohsiung Municipal Hsiao-Kang Hospital, Kaohsiung, Taiwan.
- 10Department of Pediatrics, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan.
- KMID: 2260169
- DOI: http://doi.org/10.4168/aair.2014.6.6.478
Abstract
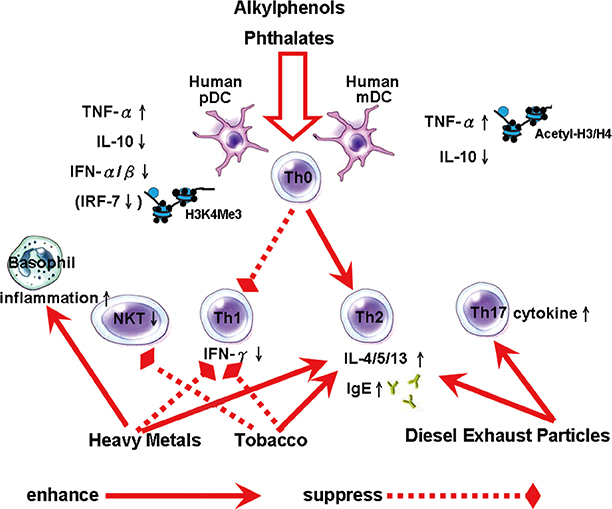
- The prevalence of asthma and allergic disease has increased worldwide over the last few decades. Many common environmental factors are associated with this increase. Several theories have been proposed to account for this trend, especially those concerning the impact of environmental toxicants. The development of the immune system, particularly in the prenatal period, has far-reaching consequences for health during early childhood, and throughout adult life. One underlying mechanism for the increased levels of allergic responses, secondary to exposure, appears to be an imbalance in the T-helper function caused by exposure to the toxicants. Exposure to environmental endocrine-disrupting chemicals can result in dramatic changes in cytokine production, the activity of the immune system, the overall Th1 and Th2 balance, and in mediators of type 1 hypersensitivity mediators, such as IgE. Passive exposure to tobacco smoke is a common risk factor for wheezing and asthma in children. People living in urban areas and close to roads with a high volume of traffic, and high levels of diesel exhaust fumes, have the highest exposure to environmental compounds, and these people are strongly linked with type 1 hypersensitivity disorders and enhanced Th2 responses. These data are consistent with epidemiological research that has consistently detected increased incidences of allergies and asthma in people living in these locations. During recent decades more than 100,000 new chemicals have been used in common consumer products and are released into the everyday environment. Therefore, in this review, we discuss the environmental effects on allergies of indoor and outside exposure.
MeSH Terms
Figure
Cited by 2 articles
-
Gene-Environment Interactions in Asthma: Genetic and Epigenetic Effects
Jong-Uk Lee, Jeong Dong Kim, Choon-Sik Park
Yonsei Med J. 2015;56(4):877-886. doi: 10.3349/ymj.2015.56.4.877.Potential health effects of emerging environmental contaminants perfluoroalkyl compounds
Youn Ju Lee
Yeungnam Univ J Med. 2018;35(2):156-164. doi: 10.12701/yujm.2018.35.2.156.
Reference
-
1. Wenzel SE, Barnes PJ, Bleecker ER, Bousquet J, Busse W, Dahlén SE, et al. A randomized, double-blind, placebo-controlled study of tumor necrosis factor-alpha blockade in severe persistent asthma. Am J Respir Crit Care Med. 2009; 179:549–558.2. Warner JO, Kaliner MA, Crisci CD, Del Giacco S, Frew AJ, Liu GH, et al. Allergy practice worldwide: a report by the World Allergy Organization Specialty and Training Council. Int Arch Allergy Immunol. 2006; 139:166–174.3. Centers for Disease Control and Prevention (US). National surveillance for asthma ---United States, 1980--2004 [Internet]. Atlanta (GA): Centers for Disease Control and Prevention;2007. cited 2012 Feb 1. Available from: http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5608a1.htm.4. Dietert RR, DeWitt JC, Germolec DR, Zelikoff JT. Breaking patterns of environmentally influenced disease for health risk reduction: immune perspectives. Environ Health Perspect. 2010; 118:1091–1099.5. Vawda S, Mansour R, Takeda A, Funnell P, Kerry S, Mudway I, et al. Associations between inflammatory and immune response genes and adverse respiratory outcomes following exposure to outdoor air pollution: a HuGE systematic review. Am J Epidemiol. 2014; 179:432–442.6. Kolarik B, Naydenov K, Larsson M, Bornehag CG, Sundell J. The association between phthalates in dust and allergic diseases among Bulgarian children. Environ Health Perspect. 2008; 116:98–103.7. Weiss ST. Eat dirt--the hygiene hypothesis and allergic diseases. N Engl J Med. 2002; 347:930–931.8. Brokken LJ, Giwercman YL. Gene-environment interactions in male reproductive health: special reference to the aryl hydrocarbon receptor signaling pathway. Asian J Androl. 2014; 16:89–96.9. Chalubinski M, Kowalski ML. Endocrine disrupters--potential modulators of the immune system and allergic response. Allergy. 2006; 61:1326–1335.10. Kato T, Tada-Oikawa S, Takahashi K, Saito K, Wang L, Nishio A, et al. Endocrine disruptors that deplete glutathione levels in APC promote Th2 polarization in mice leading to the exacerbation of airway inflammation. Eur J Immunol. 2006; 36:1199–1209.11. Giger W, Brunner PH, Schaffner C. 4-Nonylphenol in sewage sludge: accumulation of toxic metabolites from nonionic surfactants. Science. 1984; 225:623–625.12. White R, Jobling S, Hoare SA, Sumpter JP, Parker MG. Environmentally persistent alkylphenolic compounds are estrogenic. Endocrinology. 1994; 135:175–182.13. Correa-Reyes G, Viana MT, Marquez-Rocha FJ, Licea AF, Ponce E, Vazquez-Duhalt R. Nonylphenol algal bioaccumulation and its effect through the trophic chain. Chemosphere. 2007; 68:662–670.14. Fraser LR, Beyret E, Milligan SR, Adeoya-Osiguwa SA. Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum Reprod. 2006; 21:1184–1193.15. Suen JL, Hsu SH, Hung CH, Chao YS, Lee CL, Lin CY, et al. A common environmental pollutant, 4-nonylphenol, promotes allergic lung inflammation in a murine model of asthma. Allergy. 2013; 68:780–787.16. Asselin-Paturel C, Trinchieri G. Production of type I interferons: plasmacytoid dendritic cells and beyond. J Exp Med. 2005; 202:461–465.17. So EY, Park HH, Lee CE. IFN-gamma and IFN-alpha posttranscriptionally down-regulate the IL-4-induced IL-4 receptor gene expression. J Immunol. 2000; 165:5472–5479.18. Hung CH, Yang SN, Wang YF, Liao WT, Kuo PL, Tsai EM, et al. Environmental alkylphenols modulate cytokine expression in plasmacytoid dendritic cells. PLoS One. 2013; 8:e73534.19. Hung CH, Yang SN, Kuo PL, Chu YT, Chang HW, Wei WJ, et al. Modulation of cytokine expression in human myeloid dendritic cells by environmental endocrine-disrupting chemicals involves epigenetic regulation. Environ Health Perspect. 2010; 118:67–72.20. Bornehag CG, Sundell J, Weschler CJ, Sigsgaard T, Lundgren B, Hasselgren M, et al. The association between asthma and allergic symptoms in children and phthalates in house dust: a nested case-control study. Environ Health Perspect. 2004; 112:1393–1397.21. Shu H, Jönsson BA, Larsson M, Nånberg E, Bornehag CG. PVC flooring at home and development of asthma among young children in Sweden, a 10-year follow-up. Indoor Air. 2014; 24:227–235.22. Guo J, Han B, Qin L, Li B, You H, Yang J, et al. Pulmonary toxicity and adjuvant effect of di-(2-exylhexyl) phthalate in ovalbumin-immunized BALB/c mice. PLoS One. 2012; 7:e39008.23. Kuo CH, Hsieh CC, Kuo HF, Huang MY, Yang SN, Chen LC, et al. Phthalates suppress type I interferon in human plasmacytoid dendritic cells via epigenetic regulation. Allergy. 2013; 68:870–879.24. Gilmour MI, Jaakkola MS, London SJ, Nel AE, Rogers CA. How exposure to environmental tobacco smoke, outdoor air pollutants, and increased pollen burdens influences the incidence of asthma. Environ Health Perspect. 2006; 114:627–633.25. Dietert RR, Etzel RA, Chen D, Halonen M, Holladay SD, Jarabek AM, et al. Workshop to identify critical windows of exposure for children's health: immune and respiratory systems work group summary. Environ Health Perspect. 2000; 108:Suppl 3. 483–490.26. Raherison C, Pénard-Morand C, Moreau D, Caillaud D, Charpin D, Kopfersmitt C, et al. In utero and childhood exposure to parental tobacco smoke, and allergies in schoolchildren. Respir Med. 2007; 101:107–117.27. Grabenhenrich LB, Gough H, Reich A, Eckers N, Zepp F, Nitsche O, et al. Early-life determinants of asthma from birth to age 20 years: a German birth cohort study. J Allergy Clin Immunol. 2014; 133:979–988.28. Burke H, Leonardi-Bee J, Hashim A, Pine-Abata H, Chen Y, Cook DG, et al. Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics. 2012; 129:735–744.29. Lajunen TK, Jaakkola JJ, Jaakkola MS. The synergistic effect of heredity and exposure to second-hand smoke on adult-onset asthma. Am J Respir Crit Care Med. 2013; 188:776–782.30. Howrylak JA, Spanier AJ, Huang B, Peake RW, Kellogg MD, Sauers H, et al. Cotinine in children admitted for asthma and readmission. Pediatrics. 2014; 133:e355–e362.31. Moerloose KB, Robays LJ, Maes T, Brusselle GG, Tournoy KG, Joos GF. Cigarette smoke exposure facilitates allergic sensitization in mice. Respir Res. 2006; 7:49.32. Singh SP, Mishra NC, Rir-Sima-Ah J, Campen M, Kurup V, Razani-Boroujerdi S, et al. Maternal exposure to secondhand cigarette smoke primes the lung for induction of phosphodiesterase-4D5 isozyme and exacerbated Th2 responses: rolipram attenuates the airway hyperreactivity and muscarinic receptor expression but not lung inflammation and atopy. J Immunol. 2009; 183:2115–2121.33. Feng Y, Kong Y, Barnes PF, Huang FF, Klucar P, Wang X, et al. Exposure to cigarette smoke inhibits the pulmonary T-cell response to influenza virus and Mycobacterium tuberculosis. Infect Immun. 2011; 79:229–237.34. Hogan AE, Corrigan MA, O'Reilly V, Gaoatswe G, O'Connell J, Doherty DG, et al. Cigarette smoke alters the invariant natural killer T cell function and may inhibit anti-tumor responses. Clin Immunol. 2011; 140:229–235.35. Strenzke N, Grabbe J, Plath KE, Rohwer J, Wolff HH, Gibbs BF. Mercuric chloride enhances immunoglobulin E-dependent mediator release from human basophils. Toxicol Appl Pharmacol. 2001; 174:257–263.36. Nyland JF, Fillion M, Barbosa F Jr, Shirley DL, Chine C, Lemire M, et al. Biomarkers of methylmercury exposure immunotoxicity among fish consumers in Amazonian Brazil. Environ Health Perspect. 2011; 119:1733–1738.37. Patel MM, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn JW, et al. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am J Respir Crit Care Med. 2009; 180:1107–1113.38. Manners S, Alam R, Schwartz DA, Gorska MM. A mouse model links asthma susceptibility to prenatal exposure to diesel exhaust. J Allergy Clin Immunol. 2013; pii: S0091-6749(13)01709-0.39. Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013; 132:1194–1204.e2.40. Delfino RJ, Staimer N, Tjoa T, Gillen D, Kleinman MT, Sioutas C, et al. Personal and ambient air pollution exposures and lung function decrements in children with asthma. Environ Health Perspect. 2008; 116:550–558.41. Mar TF, Larson TV, Stier RA, Claiborn C, Koenig JQ. An analysis of the association between respiratory symptoms in subjects with asthma and daily air pollution in Spokane, Washington. Inhal Toxicol. 2004; 16:809–815.42. Halonen JI, Lanki T, Yli-Tuomi T, Kulmala M, Tiittanen P, Pekkanen J. Urban air pollution, and asthma and COPD hospital emergency room visits. Thorax. 2008; 63:635–641.43. Schauer JJ. Evaluation of elemental carbon as a marker for diesel particulate matter. J Expo Anal Environ Epidemiol. 2003; 13:443–453.44. Delfino RJ, Quintana PJ, Floro J, Gastañaga VM, Samimi BS, Kleinman MT, et al. Association of FEV1 in asthmatic children with personal and microenvironmental exposure to airborne particulate matter. Environ Health Perspect. 2004; 112:932–941.45. Kuroda E, Coban C, Ishii KJ. Particulate adjuvant and innate immunity: past achievements, present findings, and future prospects. Int Rev Immunol. 2013; 32:209–220.46. Aimanianda V, Haensler J, Lacroix-Desmazes S, Kaveri SV, Bayry J. Novel cellular and molecular mechanisms of induction of immune responses by aluminum adjuvants. Trends Pharmacol Sci. 2009; 30:287–295.47. Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009; 9:287–293.48. Mancino D, Buono G, Cusano M, Minucci M. Adjuvant effects of a crystalline silica on IgE and IgG1 antibody production in mice and their prevention by the macrophage stabilizer poly-2-vinylpyridine N-oxide. Int Arch Allergy Appl Immunol. 1983; 71:279–281.49. Kuroda E, Ishii KJ, Uematsu S, Ohata K, Coban C, Akira S, et al. Silica crystals and aluminum salts regulate the production of prostaglandin in macrophages via NALP3 inflammasome-independent mechanisms. Immunity. 2011; 34:514–526.50. Nygaard UC, Hansen JS, Samuelsen M, Alberg T, Marioara CD, Løvik M. Single-walled and multi-walled carbon nanotubes promote allergic immune responses in mice. Toxicol Sci. 2009; 109:113–123.51. Inoue K, Koike E, Yanagisawa R, Hirano S, Nishikawa M, Takano H. Effects of multi-walled carbon nanotubes on a murine allergic airway inflammation model. Toxicol Appl Pharmacol. 2009; 237:306–316.52. Gehring U, Wijga AH, Brauer M, Fischer P, de Jongste JC, Kerkhof M, et al. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am J Respir Crit Care Med. 2010; 181:596–603.53. Morgenstern V, Zutavern A, Cyrys J, Brockow I, Koletzko S, Krämer U, et al. Atopic diseases, allergic sensitization, and exposure to traffic-related air pollution in children. Am J Respir Crit Care Med. 2008; 177:1331–1337.54. Brauer M, Hoek G, Smit HA, de Jongste JC, Gerritsen J, Postma DS, et al. Air pollution and development of asthma, allergy and infections in a birth cohort. Eur Respir J. 2007; 29:879–888.55. Sanborn MD, Cole D, Abelsohn A, Weir E. Identifying and managing adverse environmental health effects: 4. Pesticides. CMAJ. 2002; 166:1431–1436.56. O'Malley M. Clinical evaluation of pesticide exposure and poisonings. Lancet. 1997; 349:1161–1166.57. Weselak M, Arbuckle TE, Wigle DT, Krewski D. In utero pesticide exposure and childhood morbidity. Environ Res. 2007; 103:79–86.58. Smit LA, Zuurbier M, Doekes G, Wouters IM, Heederik D, Douwes J. Hay fever and asthma symptoms in conventional and organic farmers in The Netherlands. Occup Environ Med. 2007; 64:101–107.59. Maestrelli P, Boschetto P, Fabbri LM, Mapp CE. Mechanisms of occupational asthma. J Allergy Clin Immunol. 2009; 123:531–542.