Korean J Hematol.
2012 Sep;47(3):219-224. 10.5045/kjh.2012.47.3.219.
Effects of granulocyte-colony stimulating factor and the expression of its receptor on various malignant cells
- Affiliations
-
- 1Department of Laboratory Medicine, Konkuk University School of Medicine, Seoul, Korea.
- 2Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. soonlee@plaza.snu.ac.kr
- 3Department of Laboratory Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 4Korean Cell Line Bank, Laboratory of Cell Biology, Cancer Research Center and Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2251957
- DOI: http://doi.org/10.5045/kjh.2012.47.3.219
Abstract
- BACKGROUND
Granulocyte-colony stimulating factor (G-CSF) is extensively used to improve neutrophil count during anti-cancer chemotherapy. We investigated the effects of G-CSF on several leukemic cell lines and screened for the expression of the G-CSF receptor (G-CSFR) in various malignant cells.
METHODS
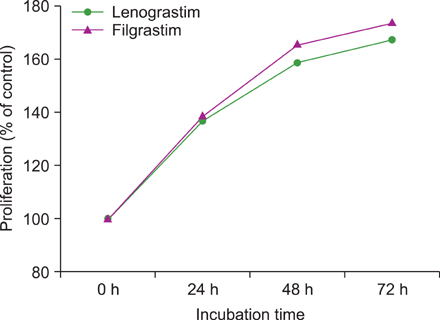
We examined the effects of the most commonly used commercial forms of G-CSF (glycosylated lenograstim and nonglycosylated filgrastim) on various leukemic cell lines by flow cytometry. Moreover, we screened for the expression of G-CSFR mRNA in 38 solid tumor cell lines by using real-time PCR.
RESULTS
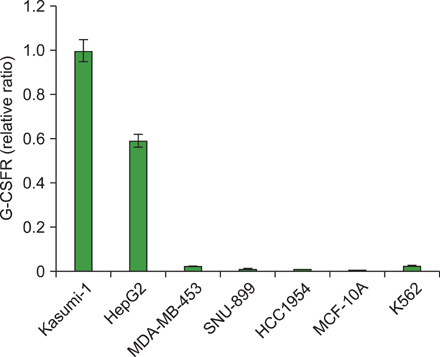
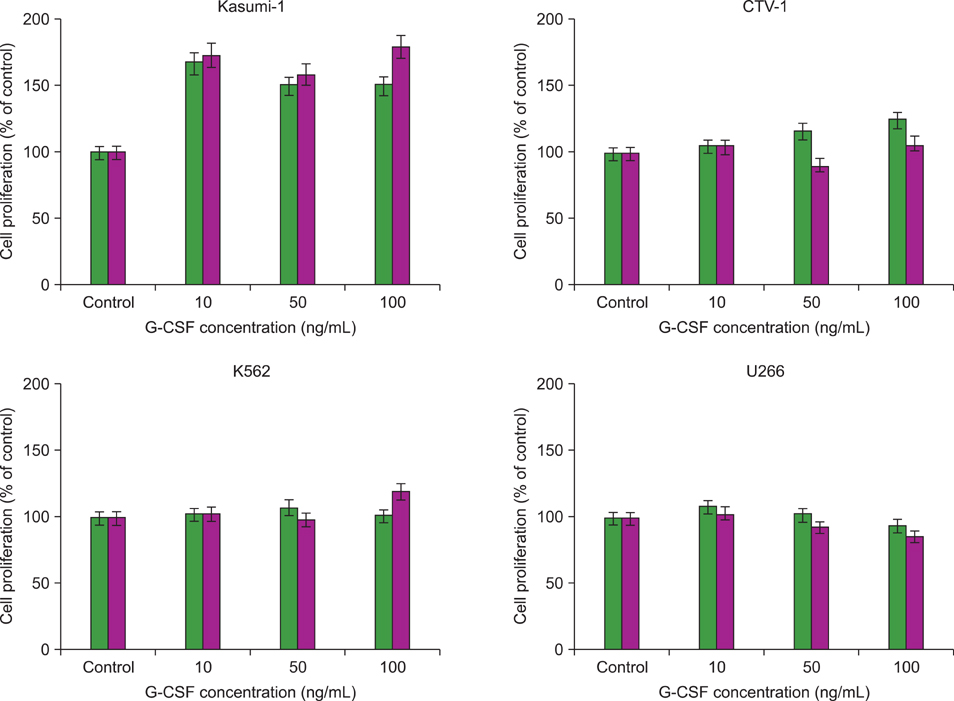
G-CSF stimulated proliferation (40-80% increase in proliferation in treated cells as compared to that in control cells) in 3 leukemic cell lines and induced differentiation of AML1/ETO+ leukemic cells. Among the 38 solid tumor cell lines, 5 cell lines (hepatoblastoma, 2 breast carcinoma, squamous cell carcinoma of the larynx, and melanoma cell lines) showed G-CSFR mRNA expression.
CONCLUSION
The results of the present study show that therapeutic G-CSF might stimulate the proliferation and differentiation of malignant cells with G-CSFR expression, suggesting that prescreening for G-CSFR expression in primary tumor cells may be necessary before using G-CSF for treatment.
Keyword
MeSH Terms
-
Breast
Carcinoma, Squamous Cell
Cell Line
Cell Line, Tumor
Flow Cytometry
Granulocyte Colony-Stimulating Factor
Larynx
Melanoma
Neutrophils
Receptors, Granulocyte Colony-Stimulating Factor
Recombinant Proteins
RNA, Messenger
Granulocyte Colony-Stimulating Factor
RNA, Messenger
Receptors, Granulocyte Colony-Stimulating Factor
Recombinant Proteins
Figure
Cited by 1 articles
-
Recombinant Human Granulocyte Colony-Stimulating Factor Promotes Preinvasive and Invasive Estrogen Receptor-Positive Tumor Development in MMTV-erbB2 Mice
Chun Ling Zhao, Guang Ping Zhang, Zheng Zheng Xiao, Zhi Kun Ma, Cai Peng Lei, Shi Yuan Song, Ying Ying Feng, Ya Chao Zhao, Xiao Shan Feng
J Breast Cancer. 2015;18(2):126-133. doi: 10.4048/jbc.2015.18.2.126.
Reference
-
1. Graf M, Hecht K, Reif S, Pelka-Fleischer R, Pfister K, Schmetzer H. Expression and prognostic value of hemopoietic cytokine receptors in acute myeloid leukemia (AML): implications for future therapeutical strategies. Eur J Haematol. 2004. 72:89–106.
Article2. Moon HW, Shin S, Kim HY, et al. Therapeutic use of granulocyte-colony stimulating factor could conceal residual malignant cells in patients with AML1/ETO+ acute myelogenous leukemia. Leukemia. 2006. 20:1408–1413.
Article3. Sung L, Nathan PC, Alibhai SM, Tomlinson GA, Beyene J. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007. 147:400–411.
Article4. Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991. 78:2791–2808.
Article5. Shimizu K, Kitabayashi I, Kamada N, et al. AML1-MTG8 leukemic protein induces the expression of granulocyte colony-stimulating factor (G-CSF) receptor through the up-regulation of CCAAT/enhancer binding protein epsilon. Blood. 2000. 96:288–296.
Article6. Hirbe AC, Uluckan O, Morgan EA, et al. Granulocyte colony-stimulating factor enhances bone tumor growth in mice in an osteoclast-dependent manner. Blood. 2007. 109:3424–3431.
Article7. Mattei S, Colombo MP, Melani C, Silvani A, Parmiani G, Herlyn M. Expression of cytokine/growth factors and their receptors in human melanoma and melanocytes. Int J Cancer. 1994. 56:853–857.
Article8. Natori T, Sata M, Washida M, Hirata Y, Nagai R, Makuuchi M. G-CSF stimulates angiogenesis and promotes tumor growth: potential contribution of bone marrow-derived endothelial progenitor cells. Biochem Biophys Res Commun. 2002. 297:1058–1061.
Article9. Noda I, Fujieda S, Ohtsubo T, et al. Granulocyte-colony-stimulating factor enhances invasive potential of human head-and-neck-carcinoma cell lines. Int J Cancer. 1999. 80:78–84.
Article10. Obermueller E, Vosseler S, Fusenig NE, Mueller MM. Cooperative autocrine and paracrine functions of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor in the progression of skin carcinoma cells. Cancer Res. 2004. 64:7801–7812.
Article11. Savarese TM, Mitchell K, McQuain C, et al. Coexpression of granulocyte colony stimulating factor and its receptor in primary ovarian carcinomas. Cancer Lett. 2001. 162:105–115.
Article12. Yang X, Liu F, Xu Z, et al. Expression of granulocyte colony stimulating factor receptor in human colorectal cancer. Postgrad Med J. 2005. 81:333–337.
Article13. Morales-Arias J, Meyers PA, Bolontrade MF, et al. Expression of granulocyte-colony-stimulating factor and its receptor in human Ewing sarcoma cells and patient tumor specimens: potential consequences of granulocyte-colony-stimulating factor administration. Cancer. 2007. 110:1568–1577.
Article14. Park HB, Yang JH, Chung KH. Characterization of the cytokine profile of platelet rich plasma (PRP) and PRP-induced cell proliferation and migration: Upregulation of matrix metalloproteinase-1 and -9 in HaCaT cells. Korean J Hematol. 2011. 46:265–273.
Article15. Ohno R, Tomonaga M, Kobayashi T, et al. Effect of granulocyte colony-stimulating factor after intensive induction therapy in relapsed or refractory acute leukemia. N Engl J Med. 1990. 323:871–877.
Article16. Heil G, Hoelzer D, Sanz MA, et al. The International Acute Myeloid Leukemia Study Group. A randomized, double-blind, placebo-controlled, phase III study of filgrastim in remission induction and consolidation therapy for adults with de novo acute myeloid leukemia. Blood. 1997. 90:4710–4718.
Article17. Godwin JE, Kopecky KJ, Head DR, et al. A double-blind placebo-controlled trial of granulocyte colony-stimulating factor in elderly patients with previously untreated acute myeloid leukemia: a Southwest oncology group study (9031). Blood. 1998. 91:3607–3615.
Article18. Usuki K, Urabe A, Masaoka T, et al. Efficacy of granulocyte colony-stimulating factor in the treatment of acute myelogenous leukaemia: a multicentre randomized study. Br J Haematol. 2002. 116:103–112.
Article19. Kroger N, Sonnenberg S, Cortes-Dericks L, Freiberger P, Mollnau H, Zander AR. Kinetics of G-CSF and CD34+ cell mobilization after once or twice daily stimulation with rHu granulocyte-stimulating factor (lenograstim) in healthy volunteers: an intraindividual crossover study. Transfusion. 2004. 44:104–110.
Article20. Hernandez-Bernal F, Garcia-García I, Gonzalez-Delgado CA, et al. Bioequivalence of two recombinant granulocyte colony-stimulating factor formulations in healthy male volunteers. Biopharm Drug Dispos. 2005. 26:151–159.
Article21. Camera A, Volpicelli M, Villa MR, Risitano AM, Rossi M, Rotoli B. Complete remission induced by high dose erythropoietin and granulocyte colony stimulating factor in acute erythroleukemia (AML-M6 with maturation). Haematologica. 2002. 87:1225–1227.22. Da Silva N, Meyer-Monard S, Menot ML, et al. Functional G-CSF pathways in t(8;21) leukemic cells allow for differentiation induction and degradation of AML1-ETO. Hematol J. 2000. 1:316–328.
Article23. Ferrara F, Di Noto R, Viola A, et al. Complete remission in acute myeloid leukaemia with t(8;21) following treatment with G-CSF: flow cytometric analysis of in vivo and in vitro effects on cell maturation. Br J Haematol. 1999. 106:520–523.
Article24. Nimubona S, Grulois I, Bernard M, et al. Complete remission in hypoplastic acute myeloid leukemia induced by G-CSF without chemotherapy: report on three cases. Leukemia. 2002. 16:1871–1873.
Article25. Takamatsu Y, Miyamoto T, Iwasaki H, Makino S, Tamura K. Remission induction by granulocyte colony-stimulating factor in hypoplastic acute myelogenous leukemia complicated by infection. A case report and review of the literature. Acta Haematol. 1998. 99:224–230.
Article26. Hernandez-Campo PM, Almeida J, Matarraz S, de Santiago M, Sanchez ML, Orfao A. Quantitative analysis of the expression of glycosylphosphatidylinositol-anchored proteins during the maturation of different hematopoietic cell compartments of normal bone marrow. Cytometry B Clin Cytom. 2007. 72:34–42.27. van Lochem EG, van der Velden VH, Wind HK, et al. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry B Clin Cytom. 2004. 60:1–13.
Article28. Ichiishi E, Yoshikawa T, Kogawa T, Yoshida N, Kondo M. Possible paracrine growth of adenocarcinoma of the stomach induced by granulocyte colony stimulating factor produced by squamous cell carcinoma of the oesophagus. Gut. 2000. 46:432–434.
Article29. Horii A, Shimamura K, Honjo Y, et al. Granulocyte colony stimulating factor-producing tongue carcinoma. Head Neck. 1997. 19:351–356.
Article30. Tachibana M, Miyakawa A, Nakashima J, et al. Autocrine growth promotion by multiple hematopoietic growth factors in the established renal cell carcinoma line KU-19-20. Cell Tissue Res. 2000. 301:353–367.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects on the production of platelet activating factor in the cultured human endothelial cells by interleukin-6 and granulocyte macrophage colony stimulating factor
- Sweet Syndrome in a Child with Aplastic Anemia after Receiving Recombinant Granulocyte Colony-stimulating Factor
- Two cases of congenital agranulocytosis treated with recombinant human granulocyte colony-stimulating factor
- The effect of granulocyte colony-stimulating factor in chemotherapy of acute myelogenous leukemia
- Plasma G-CSF and GM-CSF Concentrations and Expression of their Receptors on the Granulocyte in Children with Leukocytosis