Korean Circ J.
2008 Oct;38(10):536-543. 10.4070/kcj.2008.38.10.536.
Human Mesenchymal Stem Cell Transplantation Induces Sympathetic Nerve Sprouting and Reduces the Gap Junction With Potential Proarrhythmias in Dogs
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. hnpak@korea.ac.kr
- 2Department of Pathology, College of Medicine, Pochon CHA University, Pochon, Korea.
- 3Department of Hematology, Korea University, Seoul, Korea.
- 4Division of Cardiology, Utah Valley Medical Center, Provo, UT, USA.
- KMID: 2225718
- DOI: http://doi.org/10.4070/kcj.2008.38.10.536
Abstract
- BACKGROUND AND OBJECTIVES
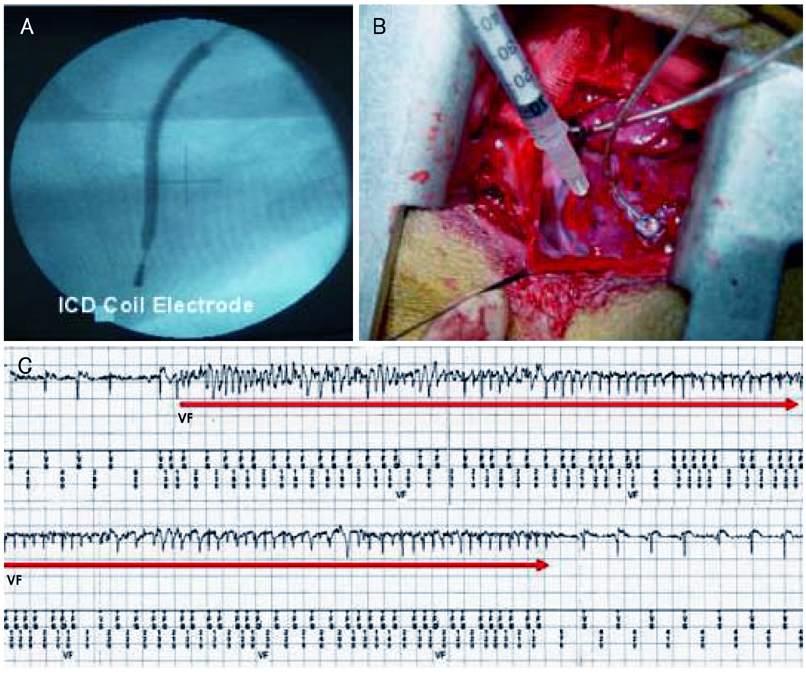
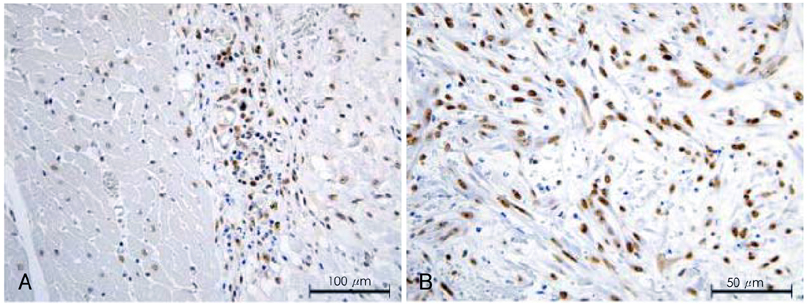
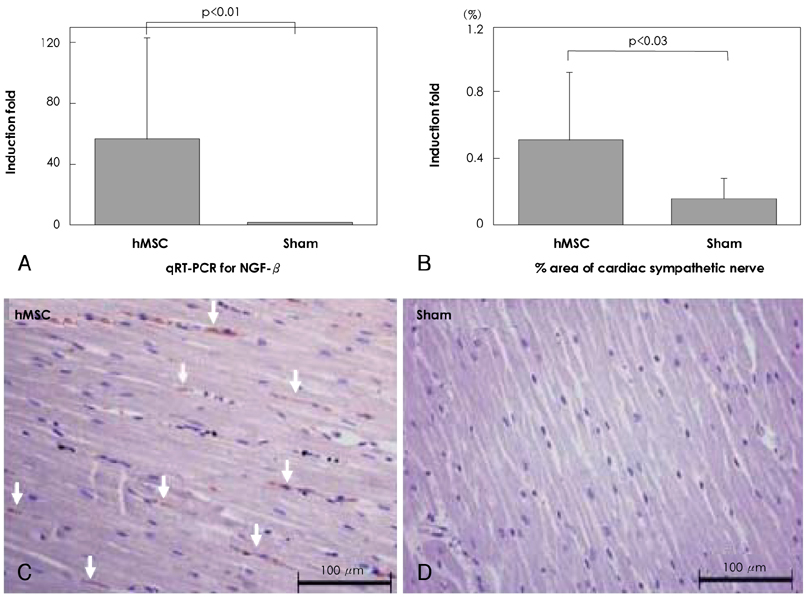
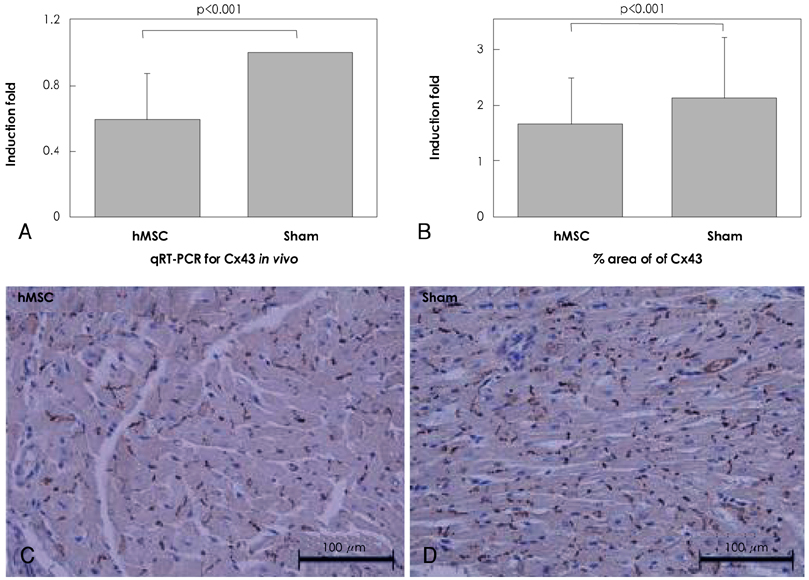
Although human mesenchymal stem cell (hMSC) transplantation has been known to improve ventricular function, the potential proarrhythmic effects have not yet been studied. MATERIALS AND METHODS: We monitored the heart rhythm of 6 dogs for 4 weeks after transplantation of hMSC (1x10(7), epicardial injection) (hMSC group) and in 5 Sham dogs after the injection of the vehicle alone. Cardiac sympathetic nerve sprouting {nerve growth factor (NGF)-beta; tyrosine hydroxylase (TH)} and gap junction expression {connexin (Cx) 43} were evaluated in 10 dogs (5 hMSC and 5 Sham) that survived longer than 4 weeks. RESULTS: The hMSC group expressed higher levels of NGF-beta messenger ribonucleic acid (mRNA) (56.0+/-66.8 fold; p<0.01) with TH+ sympathetic nerves (0.51+/-0.40 vs. 0.15+/-0.13% area; p<0.03) than the Sham control. In contrast, the hMSC group expressed lower levels of Cx43 mRNA (0.59+/-0.29 fold, p<0.001) and Cx43+ (1.64+/-1.79 vs. 2.12+/-1.07% area, p<0.001) than the Sham control. The incidences of ventricular fibrillation were 33.3% and 0% in the hMSC group and Sham control, respectively. One of the dogs with ventricular fibrillation (VF) in the hMSC group died suddenly. CONCLUSION: hMSC transplantation may be proarrhythmic since NGF-beta expression increased with cardiac sympathetic hyperinnervation and the expression of Cx43 and the gap junction decreased.
MeSH Terms
-
Animals
Connexin 43
Dogs
Gap Junctions
Heart
Humans
Incidence
Mesenchymal Stem Cell Transplantation
Mesenchymal Stromal Cells
Nerve Growth Factor
RNA
RNA, Messenger
Salicylamides
Tachycardia, Ventricular
Transplants
Tyrosine 3-Monooxygenase
Ventricular Fibrillation
Ventricular Function
Connexin 43
Nerve Growth Factor
RNA
RNA, Messenger
Salicylamides
Tyrosine 3-Monooxygenase
Figure
Reference
-
1. Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006. 355:1210–1221.2. Assmus B, Honold J, Schachinger V, et al. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006. 355:1222–1232.3. Lim D. Stem cells for cardiovascular disease. Korean Circ J. 2004. 34:435–440.4. Tse WT, Pendleton JD, Beyer WM, Egalka MC, Guinan EC. Suppression of allogeneic T-cell proliferation by human marrow stromal cells: implications in transplantation. Transplantation. 2003. 75:389–397.5. Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003. 31:890–896.6. Bartholomew A, Patil S, Mackay A, et al. Baboon mesenchymal stem cells can be genetically modified to secrete human erythropoietin in vivo. Hum Gene Ther. 2001. 12:1527–1541.7. Chang MG, Tung L, Sekar RB, et al. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006. 113:1832–1841.8. Beeres SL, Atsma DE, van der Laarse A, et al. Human adult bone marrow mesenchymal stem cells repair experimental conduction block in rat cardiomyocyte cultures. J Am Coll Cardiol. 2005. 46:1943–1952.9. Valiunas V, Doronin S, Valiuniene L, et al. Human mesenchymal stem cells make cardiac connexins and form functional gap junctions. J Physiol. 2004. 555:617–626.10. Pak HN, Qayyum M, Kim DT, et al. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003. 14:841–848.11. Cao JM, Chen LS, KenKnight BH, et al. Nerve sprouting and sudden cardiac death. Circ Res. 2000. 86:816–821.12. Cao JM, Fishbein MC, Han JB, et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation. 2000. 101:1960–1969.13. Zhou S, Chen LS, Miyauchi Y, et al. Mechanisms of cardiac nerve sprouting after myocardial infarction in dogs. Circ Res. 2004. 95:76–83.14. Liu YB, Wu CC, Lu LS, et al. Sympathetic nerve sprouting, electrical remodeling, and increased vulnerability to ventricular fibrillation in hypercholesterolemic rabbits. Circ Res. 2003. 92:1145–1152.15. Kessler PD, Byrne BJ. Myoblast cell grafting into heart muscle: cellular biology and potential applications. Ann Rev Physiol. 1999. 61:219–242.16. Liechty KW, MacKenzie TC, Shaaban AF, et al. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000. 6:1282–1286.17. Saito T, Kuang JQ, Bittira B, Al-Khaldi A, Chiu RC. Xenotransplant cardiac chimera: immune tolerance of adult stem cells. Ann Thorac Surg. 2002. 74:19–24.18. Kang HJ, Kim HS, Zhang SY, et al. Effects of intracoronary infusion of peripheral blood stem-cells mobilised with granulocyte-colony stimulating factor on left ventricular systolic function and restenosis after coronary stenting in myocardial infarction. Lancet. 2004. 363:751–756.19. Lunde K, Solheim S, Aakhus S, et al. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006. 355:1199–1209.20. Janssens S, Dubois C, Bogaert J, et al. Autologous bone marrowderived stem-cell transfer in patients with ST-segment elevation myocardial infarction: double-blind, randomised controlled trial. Lancet. 2006. 367:113–121.21. Meyer GP, Wollert KC, Lotz J, et al. Intracoronary bone marrow cell transfer after myocardial infarction: eighteen months' follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation. 2006. 113:1287–1294.22. Menasche P, Hagege AA, Vilquin JT, et al. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003. 41:1078–1083.23. Abraham MR, Henrikson CA, Tung L, et al. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005. 97:159–167.24. Fukushima S, Varela-Carver A, Coppen SR, et al. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007. 115:2254–2261.25. Shake JG, Gruber PJ, Baumgartner WA, et al. Mesenchymal stem cell implantation in a swine myocardial infarct model: engraftment and functional effects. Ann Thorac Surg. 2002. 73:1919–1925.26. Kocher AA, Schuster MD, Szabolcs MJ, et al. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001. 7:430–436.27. Orlic D, Kajstura J, Chimenti S, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001. 410:701–705.28. Fuchs S, Baffour R, Zhou YF, et al. Transendocardial delivery of autologous bone marrow enhances collateral perfusion and regional function in pigs with chronic experimental myocardial ischemia. J Am Coll Cardiol. 2001. 37:1726–1732.29. Lim SY, Jeong MH, Ahn YK, et al. The effects of mesenchymal stem cells transduced with Ark in a porcine myocardial infarction model. Korean Circ J. 2005. 35:734–741.30. Piao H, Youn TJ, Kwon JS, et al. Cellular cardiomyoplasty using bone marrow derived mesenchymal stem cells transplantation in post myocardial infarction heart failure. Korean Circ J. 2004. 34:1113–1121.31. Yang KM, Park CS, Jang SW, et al. Effect of adult bone marrow stem cells on myocardial regeneration in doxorubicin-induced mouse cardiomyopathy. Korean Circ J. 2008. 38:110–118.32. Woodbury D, Schwarz EJ, Prockop DJ, Black IB. Adult rat and human bone marrow stromal cells differentiate into neurons. J Neurosci Res. 2000. 61:364–370.33. Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003. 5:959–966.34. Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003. 425:968–973.35. Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003. 107:1164–1169.36. Martins JB, Zipes DP. Effects of sympathetic and vagal nerves on recovery properties of the endocardium and epicardium of the canine left ventricle. Circ Res. 1980. 46:100–110.37. Opthof T, Misier AR, Coronel R, et al. Dispersion of refractoriness in canine ventricular myocardium: effects of sympathetic stimulation. Circ Res. 1991. 68:1204–1215.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Safety and Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell Transplantation in Sensorineural Hearing Loss Patients
- Safety and outcomes of subconjunctival allogenic mesenchymal stem cell transplantation in canine experimental corneal defects
- Clinical utilization of cord blood over human health: experience of stem cell transplantation and cell therapy using cord blood in Korea
- Transplantation of adipose derived mesenchymal stem cells for acute thoracolumbar disc disease with no deep pain perception in dogs
- Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives