J Rheum Dis.
2012 Aug;19(4):206-211. 10.4078/jrd.2012.19.4.206.
Dietary Flavonoid Apigenin is not Effective in Preventing Development of a Bleomycin-Induced Murine Model of Scleroderma
- Affiliations
-
- 1Department of Rheumatology, Hanyang University Hospital for Rheumatic Diseases, Seoul, Korea. junjb@hanyang.ac.kr
- 2Department of Bioengineering, College of Engineering, Hanyang University, Seoul, Korea.
- 3Institute of Rheumatism, Hanyang University, Seoul, Korea.
- 4Department of Pathology, Hanyang University Medical Center, Seoul, Korea.
- 5Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 2223072
- DOI: http://doi.org/10.4078/jrd.2012.19.4.206
Abstract
OBJECTIVE
Systemic sclerosis is a connective tissue disease characterized by vasculopathy, excessive accumulation of extracellular matrix, and fibrosis of the skin and internal organs. The dietary flavonoid apigenin has been shown to reduce expression of the myofibroblast phenotype and to inhibit contraction of collagen gels. We investigated the effect of apigenin on the prevention and treatment of a modified bleomycin-induced animal model of scleroderma.
METHODS
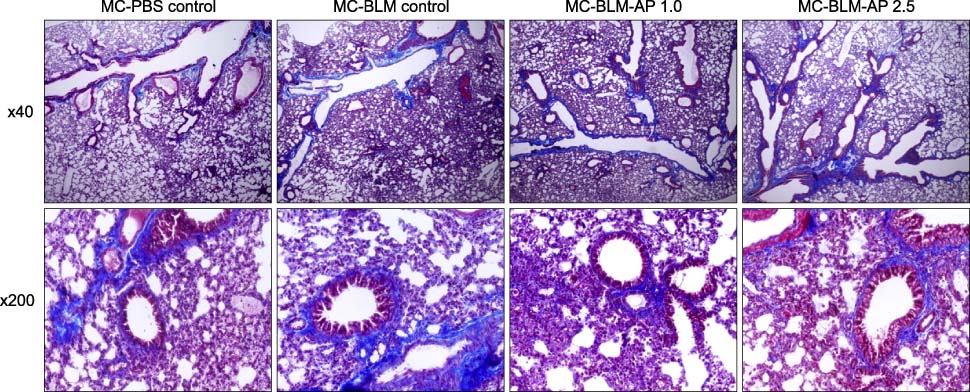
Recently, we successfully induced scleroderma by weekly subcutaneous injections of bleomycin using a thermo-reversible combination gel composed of low molecular weight methylcellulose. A weekly subcutaneous injection of methylcellulose gel loaded with bleomycin induced focal skin fibrosis on the back skin and fibrotic phenotype of lung tissue in mice. The histologic examination of skin and lungs, collagen assay of lungs, and expression of connective tissue growth factor were investigated.
RESULTS
Daily intra-peritoneal injection of 1.0 mg/kg or 2.5 mg/kg of apigenin starting a week before the bleomycin injections failed to prevent the development of skin fibrosis and reduce the fibrotic phenotypes of skin and lung tissue.
CONCLUSION
Although some in vitro experiments have supported a potential role of apigenin in the treatment of fibrosis, dietary flavonoid apigenin is not effective in preventing development of a bleomycin-induced murine model of scleroderma.
Keyword
MeSH Terms
-
Animals
Apigenin
Bleomycin
Collagen
Connective Tissue Diseases
Connective Tissue Growth Factor
Contracts
Extracellular Matrix
Fibrosis
Gels
Injections, Subcutaneous
Lung
Methylcellulose
Mice
Models, Animal
Molecular Weight
Myofibroblasts
Phenotype
Scleroderma, Systemic
Skin
Apigenin
Bleomycin
Collagen
Connective Tissue Growth Factor
Gels
Methylcellulose
Figure
Reference
-
1. LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr, et al. Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol. 1988. 15:202–205.2. Ihn H. Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci. 2008. 49:103–113.3. Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002. 22:19–34.4. Duthie G, Crozier A. Plant-derived phenolic antioxidants. Curr Opin Clin Nutr Metab Care. 2000. 3:447–451.5. Ricupero DA, Poliks CF, Rishikof DC, Kuang PP, Goldstein RH. Apigenin decreases expression of the myofibroblast phenotype. FEBS Lett. 2001. 506:15–21.6. Jun JB, Na YI, Kim TH, Yoo DH. Dietary flavonoid apigenin inhibits endothelin-1-induced contraction of collagen gel. Rheumatol Int. 2010. 30:1695–1697.7. Shi-Wen X, Denton CP, Dashwood MR, Holmes AM, Bou-Gharios G, Pearson JD, et al. Fibroblast matrix gene expression and connective tissue remodeling: role of endothelin-1. J Invest Dermatol. 2001. 116:417–425.8. Yamamoto T, Takagawa S, Katayama I, Yamazaki K, Hamazaki Y, Shinkai H, et al. Animal model of sclerotic skin. I: Local injections of bleomycin induce sclerotic skin mimicking scleroderma. J Invest Dermatol. 1999. 112:456–462.9. Jin KM, Kim YH. Injectable, thermo-reversible and complex coacervate combination gels for protein drug delivery. J Control Release. 2008. 127:249–256.10. Jun JB, Kim JK, Na YI, Jang SM, Paik SS, Kim YH. A convenient method for producing the bleomycin-induced mouse model of scleroderma by weekly injections using a methylcellulose gel. Rheumatol Int. 2012. 32:1443–1447.11. Kang HK, Ecklund D, Liu M, Datta SK. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther. 2009. 11:R59.12. Myoung HJ, Kim G, Nam KW. Apigenin isolated from the seeds of Perilla frutescens britton var crispa (Benth) inhibits food intake in C57BL/6J mice. Arch Pharm Res. 2010. 33:1741–1746.13. Ashcroft T, Simpson JM, Timbrell V. Simple method of estimating severity of pulmonary fibrosis on a numerical scale. J Clin Pathol. 1988. 41:467–470.14. Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ, Lee SK, et al. Blockade of the Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary fibrosis. Tohoku J Exp Med. 2011. 223:45–54.15. Chung MP, Monick MM, Hamzeh NY, Butler NS, Powers LS, Hunninghake GW. Role of repeated lung injury and genetic background in bleomycin-induced fibrosis. Am J Respir Cell Mol Biol. 2003. 29:375–380.16. Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993. 20:21–29.17. Hollman PC, vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB. Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med. 1996. 21:703–707.18. Cao J, Zhang Y, Chen W, Zhao X. The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr. 2010. 103:249–255.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of bleomycin-induced scleroderma
- The Therapeutic Efficacy of Apigenin in a Murine Model of Scleroderma
- Induction of Animal Model of Scleroderma with Repeated Injection of Bleomycin
- A Case of Bleomycin induced Streaky Pigmentation and Scleroderma
- Anti-fibrotic Effect of Silibinin in Keloid Fibroblasts and a Bleomycin-induced, Scleroderma-like Animal Model