J Korean Ophthalmol Soc.
2012 Dec;53(12):1864-1869. 10.3341/jkos.2012.53.12.1864.
Effect of Triamcinolone on Retinal Vessel-Related Factors in Oxygen-Induced Retinopathy Rats
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea. inyoung@gnu.ac.kr
- 2Gyeongsang Institute of Health Science, Gyeongsang National University, Jinju, Korea.
- KMID: 2216403
- DOI: http://doi.org/10.3341/jkos.2012.53.12.1864
Abstract
- PURPOSE
The present study investigated the effects of triamcinolone acetonide (TA) on retinal vessel-related factors and retinal vascular leakage in the retina of oxygen-induced retinopathy (OIR) rats.
METHODS
Sprague-Dawley rats used for OIR were exposed to hyperoxia from postnatal day 2 to day 14, then returned to normoxia from day 15 to day 30 and compared with control rats. On postnatal day 16, 2 microl of TA (4 mg/kg) was injected into the vitreous of the right eye in control and OIR rats. The Evans blue method was used for evaluating vascular leakage and RT-PCR was performed for confirmation of mRNA expression.
RESULTS
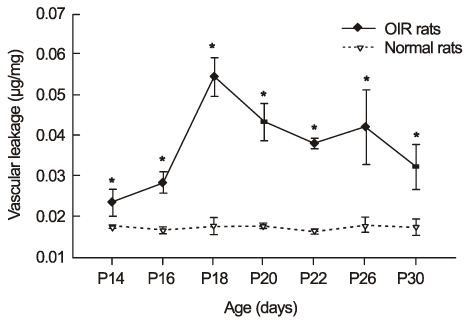
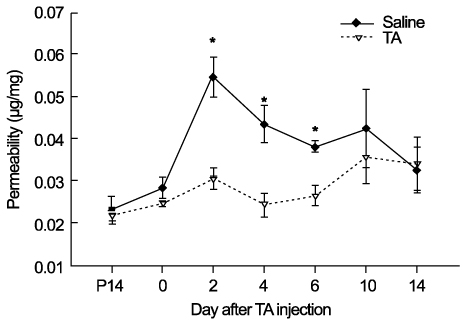
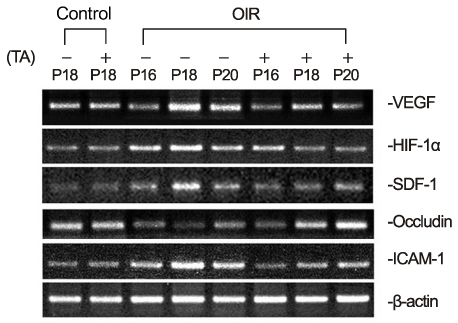
In OIR rats exposed to hyperoxia, retinal vascular permeability increased when returned to normoxia. After intravitreal injection of TA, vascular permeability was decreased in OIR rats, but no changes were found in control rats. In OIR rats, mRNAs of HIF-1alpha, VEGF, SDF-1 and ICAM-1 were more expressed and down-regulated after intravitreal injection of TA. Occludin mRNA level was decreased in the hypoxic condition and up-regulated after TA treatment.
CONCLUSIONS
The present study suggests the ability of TA to inhibit retinal vascular leakage in OIR rats and a possibility that TA controls expression of the HIF-1, VEGF, SDF-1, ICAM-1 and Occludin, which may protect retinal vascular destruction from hypoxic conditions; further studies are necessary to confirm changes in protein levels.
Keyword
MeSH Terms
-
Animals
Capillary Permeability
Evans Blue
Eye
Hyperoxia
Intercellular Adhesion Molecule-1
Intravitreal Injections
Occludin
Rats
Rats, Sprague-Dawley
Retina
Retinaldehyde
RNA, Messenger
Triamcinolone
Triamcinolone Acetonide
Vascular Endothelial Growth Factor A
Evans Blue
Intercellular Adhesion Molecule-1
Occludin
RNA, Messenger
Retinaldehyde
Triamcinolone
Triamcinolone Acetonide
Vascular Endothelial Growth Factor A
Figure
Cited by 1 articles
-
Effect of Posterior Subtenon Triamcinolone Injection during Vitrectomy for Idiopathic Epiretinal Membrane
Sung Il Kim, Sung Who Park, Ik Soo Byon, Ji Eun Lee
J Korean Ophthalmol Soc. 2015;56(8):1236-1241. doi: 10.3341/jkos.2015.56.8.1236.
Reference
-
1. Hebbandi SB, Bowen JR, Hipwell GC, et al. Ocular sequelae in extremely premature infants at 5 years of age. J Paediatr Child Health. 1997. 33:339–342.2. Moore A. Retinopathy of prematurity. 1990. Boston: Blackwell Scientific;365–375.3. Noma H, Funatsu H, Yamashita H, et al. Regulation of angiogenesis in diabetic retinopathy: possible balance between vascular endothelial growth factor and endostatin. Arch Ophthalmol. 2002. 120:1075–1080.4. Raisler BJ, Berns KI, Grant MB, et al. Adeno-associated virus type-2 expression of pigmented epithelium-derived factor or Kringles 1-3 of angiostatin reduce retinal neovascularization. Proc Natl Acad Sci U S A. 2002. 99:8909–8914.5. Adamis AP, Miller JW, Bernal MT, et al. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994. 118:445–450.6. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994. 331:1480–1487.7. Malecaze F, Clamens S, Simorre-Pinatel V, et al. Detection of vascular endothelial growth factor messenger RNA and vascular endothelial growth factor-like activity in proliferative diabetic retinopathy. Arch Ophthalmol. 1994. 112:1476–1482.8. Brafman A, Mett I, Shafir M, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci. 2004. 45:3796–3805.9. Treins C, Giorgetti-Peraldi S, Murdaca J, Van Obberghen E. Regulation of vascular endothelial growth factor expression by advanced glycation end products. J Biol Chem. 2001. 276:43836–43841.10. Poulaki V, Qin W, Joussen AM, et al. Acute intensive insulin therapy exacerbates diabetic blood-retinal barrier breakdown via hypoxia-inducible factor-1alpha and VEGF. J Clin Invest. 2002. 109:805–815.11. Grimm C, Wenzel A, Groszer M, et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nat Med. 2002. 8:718–724.12. Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006. 124:175–189.13. Lima e Silva R, Shen J, Hackett SF, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007. 21:3219–3230.14. Antonetti DA, Barber AJ, Khin S, et al. Penn State Retina Research Group. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998. 47:1953–1959.15. McAllister IL, Vijayasekaran S, Chen SD, Yu DY. Effect of triamcinolone acetonide on vascular endothelial growth factor and occludin levels in branch retinal vein occlusion. Am J Ophthalmol. 2009. 147:838–846. 846.e1–846.e2.16. Kim I, Moon SO, Kim SH, et al. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J Biol Chem. 2001. 276:7614–7620.17. Joussen AM, Poulaki V, Qin W, et al. Retinal vascular endothelial growth factor induces intercellular adhesion molecule-1 and endothelial nitric oxide synthase expression and initiates early diabetic retinal leukocyte adhesion in vivo. Am J Pathol. 2002. 160:501–509.18. Clauss M, Gerlach M, Gerlach H, et al. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990. 172:1535–1545.19. Barleon B, Sozzani S, Zhou D, et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood. 1996. 87:3336–3343.20. Sunderkötter C, Steinbrink K, Goebeler M, et al. Macrophages and angiogenesis. J Leukoc Biol. 1994. 55:410–422.21. Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004. 25:581–611.22. Sakurai E, Taguchi H, Anand A, et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003. 44:2743–2749.23. Tatar O, Adam A, Shinoda K, et al. Early effects of intravitreal triamcinolone acetonide on inflammation and proliferation in human choroidal neovascularization. Arch Ophthalmol. 2009. 127:275–281.24. Kim YH, Chung IY, Choi MY, et al. Triamcinolone suppresses retinal vascular pathology via a potent interruption of proinflammatory signal-regulated activation of VEGF during a relative hypoxia. Neurobiol Dis. 2007. 26:569–576.25. Antcliff RJ, Spalton DJ, Stanford MR, et al. Intravitreal triamcinolone for uveitic cystoid macular edema: an optical coherence tomography study. Ophthalmology. 2001. 108:765–772.26. Young S, Larkin G, Branley M, Lightman S. Safety and efficacy of intravitreal triamcinolone for cystoid macular oedema in uveitis. Clin Experiment Ophthalmol. 2001. 29:2–6.27. Jonas JB, Söfker A. Intraocular injection of crystalline cortisone as adjunctive treatment of diabetic macular edema. Am J Ophthalmol. 2001. 132:425–427.28. Jonas JB, Hayler JK, Panda-Jonas S. Intravitreal injection of crystalline cortisone as adjunctive treatment of proliferative vitreoretinopathy. Br J Ophthalmol. 2000. 84:1064–1067.29. Danis RP, Ciulla TA, Pratt LM, Anliker W. Intravitreal triamcinolone acetonide in exudative age-related macular degeneration. Retina. 2000. 20:244–250.30. Challa JK, Gillies MC, Penfold PL, et al. Exudative macular degeneration and intravitreal triamcinolone: 18 month follow up. Aust N Z J Ophthalmol. 1998. 26:277–281.31. Xu Q, Qaum T, Adamis AP. Sensitive blood-retinal barrier breakdown quantitation using Evans blue. Invest Ophthalmol Vis Sci. 2001. 42:789–794.32. Kim YS, Kim YH, Cheon EW, et al. Retinal expression of clusterin in the streptozotocin-induced diabetic rat. Brain Res. 2003. 976:53–59.33. Shih SC, Ju M, Liu N, Smith LE. Selective stimulation of VEGFR-1 prevents oxygen-induced retinal vascular degeneration in retinopathy of prematurity. J Clin Invest. 2003. 112:50–57.34. Leske DA, Wu J, Fautsch MP, et al. The role of VEGF and IGF-1 in a hypercarbic oxygen-induced retinopathy rat model of ROP. Mol Vis. 2004. 10:43–50.35. Zhang SX, Ma JX, Sima J, et al. Genetic difference in susceptibility to the blood-retina barrier breakdown in diabetes and oxygen-induced retinopathy. Am J Pathol. 2005. 166:313–321.36. Gao G, Shao C, Zhang SX, et al. Kallikrein-binding protein inhibits retinal neovascularization and decreases vascular leakage. Diabetologia. 2003. 46:689–698.37. Ozaki H, Hayashi H, Vinores SA, et al. Intravitreal sustained release of VEGF causes retinal neovascularization in rabbits and breakdown of the blood-retinal barrier in rabbits and primates. Exp Eye Res. 1997. 64:505–517.38. Saishin Y, Saishin Y, Takahashi K, et al. VEGF-TRAP(R1R2) suppresses choroidal neovascularization and VEGF-induced breakdown of the blood-retinal barrier. J Cell Physiol. 2003. 195:241–248.39. Nambu H, Nambu R, Oshima Y, et al. Angiopoietin 1 inhibits ocular neovascularization and breakdown of the blood-retinal barrier. Gene Ther. 2004. 11:865–873.40. Houle JM, Strong HA. Duration of skin photosensitivity and incidence of photosensitivity reactions after administration of verteporfin. Retina. 2002. 22:691–697.41. Brooks HL Jr, Caballero S Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004. 122:1801–1807.42. Danis RP, Bingaman DP, Yang Y, Ladd B. Inhibition of preretinal and optic nerve head neovascularization in pigs by intravitreal triamcinolone acetonide. Ophthalmology. 1996. 103:2099–2104.43. Jonas JB, Kreissig I, Kamppeter B, Degenring RF. [Intravitreal triamcinolone acetonide for the treatment of intraocular edematous and neovascular diseases]. Ophthalmologe. 2004. 101:113–120.44. Lai DR, Chen HR, Lin LM, et al. Clinical evaluation of different treatment methods for oral submucous fibrosis. A 10-year experience with 150 cases. J Oral Pathol Med. 1995. 24:402–406.45. Matsuda S, Gomi F, Oshima Y, et al. Vascular endothelial growth factor reduced and connective tissue growth factor induced by triamcinolone in ARPE19 cells under oxidative stress. Invest Ophthalmol Vis Sci. 2005. 46:1062–1068.46. Lipworth BJ. Systemic adverse effects of inhaled corticosteroid therapy: A systematic review and meta-analysis. Arch Intern Med. 1999. 159:941–955.47. Takahashi K, Saishin Y, Saishin Y, et al. Intraocular expression of endostatin reduces VEGF-induced retinal vascular permeability, neovascularization, and retinal detachment. FASEB J. 2003. 17:896–898.48. Sima J, Zhang SX, Shao C, et al. The effect of angiostatin on vascular leakage and VEGF expression in rat retina. FEBS Lett. 2004. 564:19–23.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Triamcinolone Acetonide Prevents Enhancement of Hypoxia-induced Neuronal and Inducible Nitric Oxide Synthases in the Retinas of Rats with Oxygen-induced Retinopathy
- Retinal Vascular Caliber Changes in Diabetic Retinopathy after Panretinal Photocoagulation and Additive Bevacizumab Injections
- Effect of Curcumin in a Mouse Model of Oxygen-Induced Retinopathy
- An Ultrastructural Study on the Early Morphologic Changes of the Retina in Streptozotocin-induced Diabetic Rats
- Ultrastructural Changes of The Retinal Capillary Basement Membrane in The Streptozotocin-Induced Diabetic Rats