Korean J Ophthalmol.
2012 Dec;26(6):455-461. 10.3341/kjo.2012.26.6.455.
Triamcinolone Acetonide Prevents Enhancement of Hypoxia-induced Neuronal and Inducible Nitric Oxide Synthases in the Retinas of Rats with Oxygen-induced Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, Gyeongsang National University School of Medicine, Jinju, Korea. YJM@nongae.gsnu.ac.kr
- 2Institute of Health Science, Gyeongsang National University, Jinju, Korea.
- 3Department of Anatomy and Neurobiology, BK21 Biomedical Center, Gyeongsang National University School of Medicine, Jinju, Korea.
- KMID: 1499685
- DOI: http://doi.org/10.3341/kjo.2012.26.6.455
Abstract
- PURPOSE
We investigated whether oxygen-induced retinopathy (OIR) results in changes in the protein expression of neuronal and inducible nitric oxide synthases (nNOS and iNOS, respectively) in rat model of OIR. In addition, we evaluated whether treatment of rats with triamcinolone acetonide (TA) prevents this response.
METHODS
To promote OIR, Sprague-Dawley rats were exposed to hyperoxia from postnatal day 2 (P2) to P14. They were then returned to normoxia after P15. TA was injected into the right vitreous of P15 rats, while saline was injected into the left vitreous. At P18 the expression of nNOS and iNOS was determined using Western blotting and immunostaining techniques in retinas obtained from control rats.
RESULTS
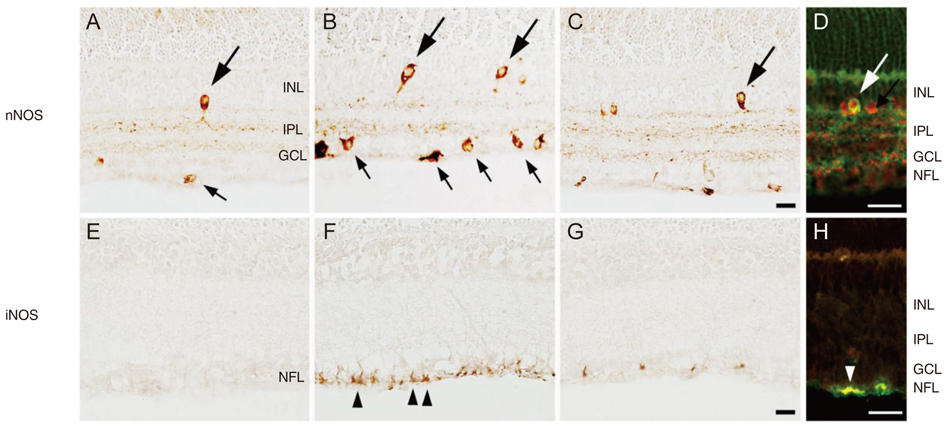
In P18 OIR rats, the abundance of nNOS and iNOS protein was significantly increased compared with controls. These increases were not observed in the retinas of rats treated with TA. The change in expression of nNOS and iNOS were specific to parvalbumin and glial fibrillary acidic protein-positive cells. Treatment with TA prevented the increased expression of nNOS and iNOS in all samples.
CONCLUSIONS
Hypoxia upregulates expression of nNOS and iNOS in OIR rat retinas, which is can be prevented by treatment with TA.
Keyword
MeSH Terms
-
Animals
Animals, Newborn
Anoxia/metabolism/pathology/*prevention & control
Blotting, Western
Disease Models, Animal
Female
Glucocorticoids/pharmacology
Immunohistochemistry
Neurons/metabolism
Nitric Oxide Synthase Type II/*biosynthesis
Oxygen/toxicity
Pregnancy
*Pregnancy, Animal
Rats
Rats, Sprague-Dawley
Retina/*metabolism/pathology
Retinal Diseases/chemically induced/pathology/*prevention & control
Triamcinolone Acetonide/*pharmacology
Glucocorticoids
Triamcinolone Acetonide
Oxygen
Nitric Oxide Synthase Type II
Figure
Reference
-
1. Brafman A, Mett I, Shafir M, et al. Inhibition of oxygen-induced retinopathy in RTP801-deficient mice. Invest Ophthalmol Vis Sci. 2004. 45:3796–3805.2. Hammes HP, Lin J, Renner O, et al. Pericytes and the pathogenesis of diabetic retinopathy. Diabetes. 2002. 51:3107–3112.3. Inatani M, Tanihara H, Honjo M, et al. Expression of proteoglycan decorin in neural retina. Invest Ophthalmol Vis Sci. 1999. 40:1783–1791.4. Ishida S, Usui T, Yamashiro K, et al. VEGF164-mediated inflammation is required for pathological, but not physiological, ischemia-induced retinal neovascularization. J Exp Med. 2003. 198:483–489.5. Downie LE, Pianta MJ, Vingrys AJ, et al. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J Comp Neurol. 2007. 504:404–417.6. Kim YH, Chung IY, Choi MY, et al. Triamcinolone suppresses retinal vascular pathology via a potent interruption of proinflammatory signal-regulated activation of VEGF during a relative hypoxia. Neurobiol Dis. 2007. 26:569–576.7. Dawson VL, Dawson TM. Nitric oxide neurotoxicity. J Chem Neuroanat. 1996. 10:179–190.8. Dawson VL, Dawson TM, London ED, et al. Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A. 1991. 88:6368–6371.9. Kaur C, Sivakumar V, Foulds WS, et al. Cellular and vascular changes in the retina of neonatal rats after an acute exposure to hypoxia. Invest Ophthalmol Vis Sci. 2009. 50:5364–5374.10. Kaur C, Sivakumar V, Foulds WS. Early response of neurons and glial cells to hypoxia in the retina. Invest Ophthalmol Vis Sci. 2006. 47:1126–1141.11. Almeida A, Heales SJ, Bolanos JP, Medina JM. Glutamate neurotoxicity is associated with nitric oxide-mediated mitochondrial dysfunction and glutathione depletion. Brain Res. 1998. 790:209–216.12. Osborne NN, Casson RJ, Wood JP, et al. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004. 23:91–147.13. Chemtob S, Hardy P, Abran D, et al. Peroxide-cyclooxygenase interactions in postasphyxial changes in retinal and choroidal hemodynamics. J Appl Physiol. 1995. 78:2039–2046.14. Hardy P, Dumont I, Bhattacharya M, et al. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000. 47:489–509.15. Rey-Funes M, Ibarra ME, Dorfman VB, et al. Hypothermia prevents nitric oxide system changes in retina induced by severe perinatal asphyxia. J Neurosci Res. 2011. 89:729–743.16. Lee EJ, Kim KY, Gu TH, et al. Neuronal nitric oxide synthase is expressed in the axotomized ganglion cells of the rat retina. Brain Res. 2003. 986:174–180.17. Abraham IM, Harkany T, Horvath KM, Luiten PG. Action of glucocorticoids on survival of nerve cells: promoting neurodegeneration or neuroprotection? J Neuroendocrinol. 2001. 13:749–760.18. Jonas JB, Kreissig I, Degenring R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog Retin Eye Res. 2005. 24:587–611.19. Ozdek SC, Aydin B, Gurelik G, et al. Effects of intravitreal triamcinolone injection on macular edema and visual prognosis in central retinal vein occlusion. Int Ophthalmol. 2005. 26:27–34.20. Psarra AM, Bochaton-Piallat ML, Gabbiani G, et al. Mitochondrial localization of glucocortocoid receptor in glial (Müller) cells in the salamander retina. Glia. 2003. 41:38–49.21. Hartnett ME, Martiniuk DJ, Saito Y, et al. Triamcinolone reduces neovascularization, capillary density and IGF-1 receptor phosphorylation in a model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 2006. 47:4975–4982.22. Park YJ, Kim YH, Choi WS, et al. Treatment with triamcinolone acetonide prevents decreased retinal levels of decorin in a rat model of oxygen-induced retinopathy. Curr Eye Res. 2010. 35:657–663.23. Park JW, Park SJ, Park SH, et al. Up-regulated expression of neuronal nitric oxide synthase in experimental diabetic retina. Neurobiol Dis. 2006. 21:43–49.24. Park SH, Kim JH, Kim YH, Park CK. Expression of neuronal nitric oxide synthase in the retina of a rat model of chronic glaucoma. Vision Res. 2007. 47:2732–2740.25. Leal EC, Manivannan A, Hosoya K, et al. Inducible nitric oxide synthase isoform is a key mediator of leukostasis and blood-retinal barrier breakdown in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2007. 48:5257–5265.26. Kim WT, Suh ES. Retinal protective effects of resveratrol via modulation of nitric oxide synthase on oxygen-induced retinopathy. Korean J Ophthalmol. 2010. 24:108–118.27. Jacobson LK, Dutton GN. Periventricular leukomalacia: an important cause of visual and ocular motility dysfunction in children. Surv Ophthalmol. 2000. 45:1–13.28. Bredt DS, Snyder SH. Nitric oxide, a novel neuronal messenger. Neuron. 1992. 8:3–11.29. Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991. 43:109–142.30. Raju TN, Langenberg P, Bhutani V, Quinn GE. Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal of published trials. J Pediatr. 1997. 131:844–850.31. Rotschild T, Nandgaonkar BN, Yu K, Higgins RD. Dexamethasone reduces oxygen induced retinopathy in a mouse model. Pediatr Res. 1999. 46:94–100.32. Wenzel A, Grimm C, Seeliger MW, et al. Prevention of photoreceptor apoptosis by activation of the glucocorticoid receptor. Invest Ophthalmol Vis Sci. 2001. 42:1653–1659.33. Gwon JS, Ju WK, Park SJ, et al. The regulatory expression of neuronal nitric oxide synthase in the ischemic rat retina. Neuroreport. 2001. 12:3385–3389.34. Bolanos JP, Almeida A. Roles of nitric oxide in brain hypoxia-ischemia. Biochim Biophys Acta. 1999. 1411:415–436.35. Oh SJ, Kim IB, Lee MY, et al. NOS-like immunoreactive neurons express GABA-like immunoreactivity in rabbit and rat retinae. Exp Brain Res. 1998. 120:109–113.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Triamcinolone Acetonide on the Survival and Nitric Oxide Production in Cultured Human Tenon's Capsule Fibroblasts
- Effect of Triamcinolone on Retinal Vessel-Related Factors in Oxygen-Induced Retinopathy Rats
- Role of Nitric Oxide in the Proliferative and Migratory Effect of Triamcinolone in RPE Cells
- The Effects of Resveratrol via Mediation of Nitric Oxide Synthase (NOS) on Hypoxic Retinal Injury in Neonatal Rats
- Retinal Protective Effects of Resveratrol via Modulation of Nitric Oxide Synthase on Oxygen-induced Retinopathy