J Lung Cancer.
2008 Dec;7(2):65-70. 10.6058/jlc.2008.7.2.65.
Detection of Serum Free DNA Hypermethylation in Surgically Resected Adenocarcinoma of the Lung
- Affiliations
-
- 1Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea. ytkim@snu.ac.kr
- 2Transplantation Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Thoracic and Cardiovascular Surgery, Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- KMID: 2200028
- DOI: http://doi.org/10.6058/jlc.2008.7.2.65
Abstract
-
PURPOSE : Aberrant DNA methylation patterns have been commonly associated with human cancers. We have investigated the frequency of DNA hypermethylation in promoter regions from adenocarcinomas of the lung and then attempted to detect the same epigenetic changes from patient serum samples.
MATERIALS AND METHODS
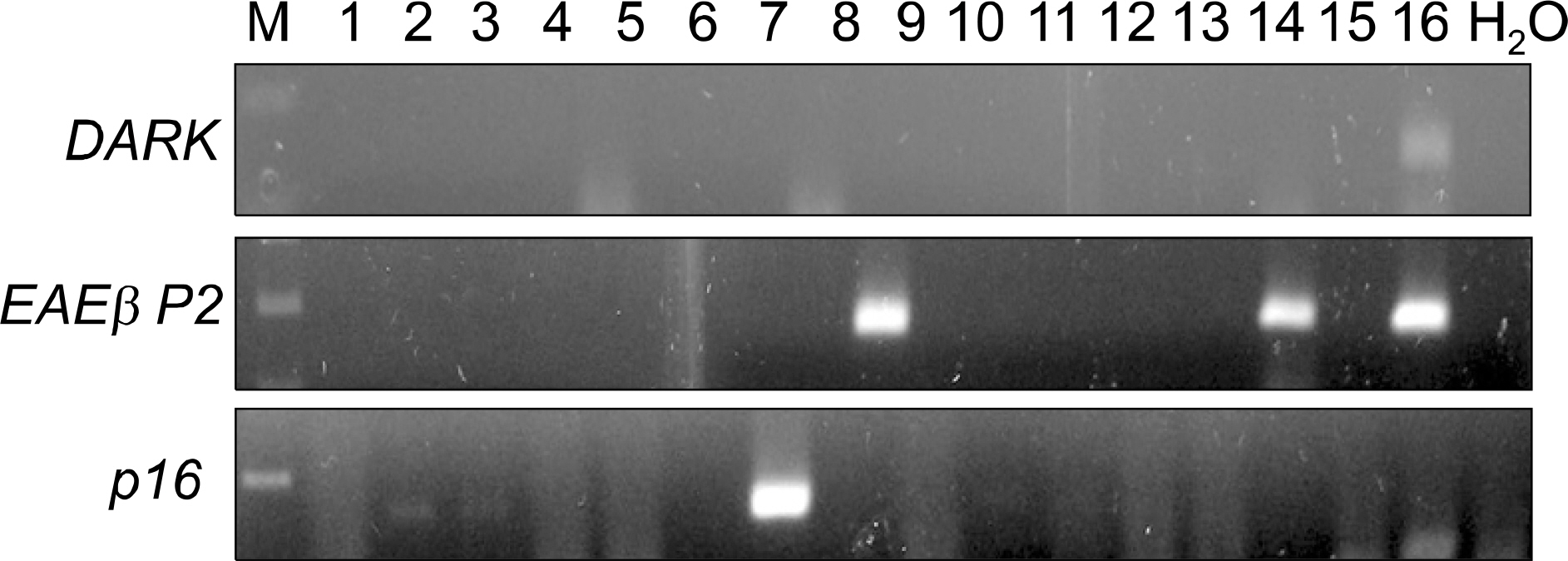
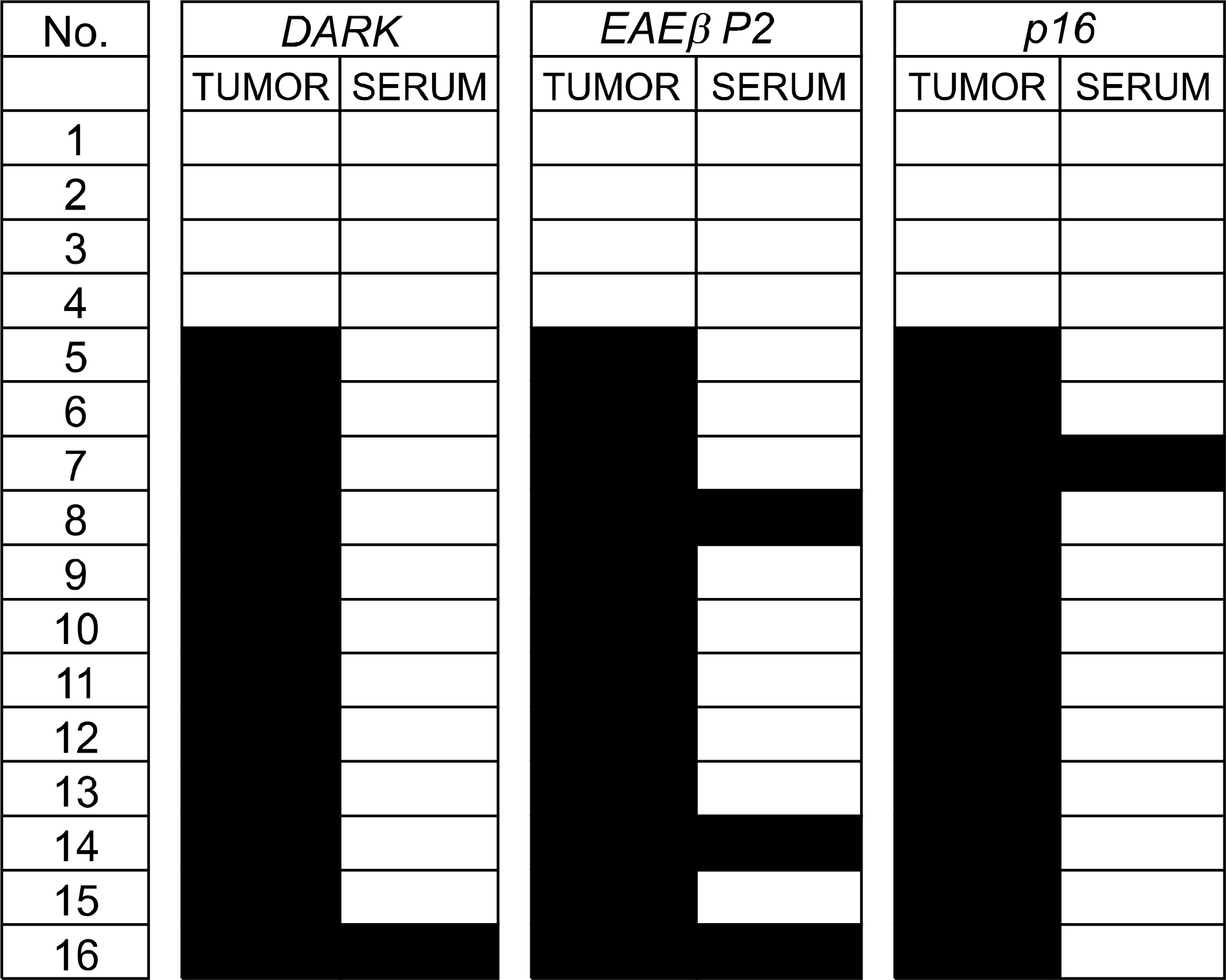
: We collected tissues from 72 cases of lung adenocarcinomas. The cancer and normal lung tissues were tested for DNA hypermethylation using methylation-specific PCR (MSP). The genes investigated were DAPK, RARbetaP2 and p16. We selected 12 patients where promoter hypermethylation was present for all three genes and four patients where hypermethylation was not seen for any of the three genes. Serum-free DNA was extracted and was tested for promoter hypermethylation. The status of serum-free DNA methylation was analyzed; the hypermethylation status was compared to clinical variables and cancer outcomes.
RESULTS
: DNA hypermethylation was observed in 32% of samples for DAPK, 63% of samples for RARbetaP2 and 83% of samples for p16 from the cancer tissues. Among the 12 matched serum samples where the primary tumor showed hypermethylation in all three gene promoter regions, we were able to detect five incidences of serum DNA hypermethylation in four patients. The four patients had TNM stage II or higher disease. None of the patients with stage I disease showed serum-free DNA hypermethylation.
CONCLUSION
: Aberrant promoter hypermethylation was frequently observed in surgically resected adenocarcinoma of the lung. Concurrent serum-free DNA hypermethylation was detected in 34% of patients where the primary tumor showed hypermethylation in all three gene promoter regions. The findings suggest that the serum-free DNA methylation status might be used as a potential target for the diagnosis of lung cancer. However, the low sensitivity should be improved for use in a clinical application
Keyword
MeSH Terms
Figure
Reference
-
References
1. Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999; 49:8–31.
Article2. Bunn PJ Jr. Early detection of lung cancer using serum RNA or DNA markers: ready for "prime time" or for validation? J Clin Oncol. 2003; 21:3891–3893.
Article3. Kim YT, Lee SH, Sung SW, Kim JH. Can aberrant promoter hypermethylation of CpG islands predict the clinical outcome of nonsmall cell lung cancer after curative resection? Ann Thorac Surg. 2005; 79:1180–1188.
Article4. Kim YT, Park SJ, Lee SH, et al. Prognostic implication of aberrant promoter hypermethylation of CpG islands in adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2005; 130:1378.
Article5. Jahr S, Hentze H, Englisch S, et al. DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001; 61:1659–1665.6. Sozzi G, Conte D, Leon M, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol. 2003; 21:3902–3908.
Article7. Sozzi G, Conte D, Mariani L, et al. Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res. 2001; 61:4675–4678.8. Bearzatto A, Conte D, Frattini M, et al. p16 (INK4A) Hypermethylation detected by fluorescent methylation-specific PCR in plasmas from nonsmall cell lung cancer. Clin Cancer Res. 2002; 8:3782–3787.9. Beau-Faller M, Gaub MP, Schneider A, et al. Plasma DNA microsatellite panel as sensitive and tumor-specific marker in lung cancer patients. Int J Cancer. 2003; 105:361–370.
Article10. Gormally E, Hainaut P, Caboux E, et al. Amount of DNA in plasma and cancer risk: a prospective study. Int J Cancer. 2004; 111:746–749.
Article11. Gautschi O, Bigosch C, Huegli B, et al. Circulating deoxyribonucleic Acid as prognostic marker in nonsmall-cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004; 22:4157–4164.
Article12. Herrera LJ, Raja S, Gooding WE, et al. Quantitative analysis of circulating plasma DNA as a tumor marker in thoracic malignancies. Clin Chem. 2005; 51:113–118.
Article13. Chen XQ, Stroun M, Magnenat JL, et al. Microsatellite alterations in plasma DNA of small cell lung cancer patients. Nat Med. 1996; 2:1033–1035.
Article14. Sozzi G, Musso K, Ratcliffe C, Goldstraw P, Pierotti MA, Pastorino U. Detection of microsatellite alterations in plasma DNA of nonsmall cell lung cancer patients: a prospect for early diagnosis. Clin Cancer Res. 1999; 5:2689–2692.15. Andriani F, Conte D, Mastrangelo T, et al. Detecting lung cancer in plasma with the use of multiple genetic markers. Int J Cancer. 2004; 108:91–96.
Article16. Cuda G, Gallelli A, Nistico A, et al. Detection of microsatellite instability and loss of heterozygosity in serum DNA of small and nonsmall cell lung cancer patients: a tool for early diagnosis? Lung Cancer. 2000; 30:211–214.
Article17. Ramirez JL, Sarries C, de Castro PL, et al. Methylation patterns and K-ras mutations in tumor and paired serum of resected nonsmall-cell lung cancer patients. Cancer Lett. 2003; 193:207–216.
Article18. Kimura T, Holland WS, Kawaguchi T, et al. Mutant DNA in plasma of lung cancer patients: potential for monitoring response to therapy. Ann N Y Acad Sci. 2004; 1022:55–60.
Article19. Silva JM, Gonzalez R, Dominguez G, Garcia JM, Espana P, Bonilla F. TP53 gene mutations in plasma DNA of cancer patients. Genes Chromosomes Cancer. 1999; 24:160–161.
Article20. An Q, Liu Y, Gao Y, et al. Detection of p16 hypermethylation in circulating plasma DNA of nonsmall cell lung cancer patients. Cancer Lett. 2002; 188:109–114.
Article21. Esteller M, Sanchez-Cespedes M, Rosell R, Sidransky D, Baylin SB, Herman JG. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from nonsmall cell lung cancer patients. Cancer Res. 1999; 59:67–70.22. Usadel H, Brabender J, Danenberg KD, et al. Quantitative adenomatous polyposis coli promoter methylation analysis in tumor tissue, serum, and plasma DNA of patients with lung cancer. Cancer Res. 2002; 62:371–375.23. Xue X, Zhu YM, Woll PJ. Circulating DNA and lung cancer. Ann N Y Acad Sci. 2006; 1075:154–164.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- DNA Hypermethylation and the Loss of Heterozygosity of Chromosome 16q22 in Hepatocellular Carcinoma
- No Detection of Episomal or Integrated High-Risk Human Papillomavirus in Nonsmall Cell Lung Carcinomas among Korean Population
- Epigenetic Changes (Aberrant DNA Methylation) in Colorectal Neoplasia
- The Overexpression of Histone Deacetylase 1 and Its Relationship with p16INK4a Gene Hypermethylation in Pulmonary Squamous Cell Carcinoma and Adenocarcinoma
- Hypermethylation of p16(INK4a) in Korean Non-small Cell Lung Cancer Patients