J Korean Med Assoc.
2009 Jul;52(7):665-676. 10.5124/jkma.2009.52.7.665.
Multiple Sclerosis
- Affiliations
-
- 1Department of Neurology, National Cancer Center, Korea. hojinkim@ncc.re.kr
- KMID: 2188027
- DOI: http://doi.org/10.5124/jkma.2009.52.7.665
Abstract
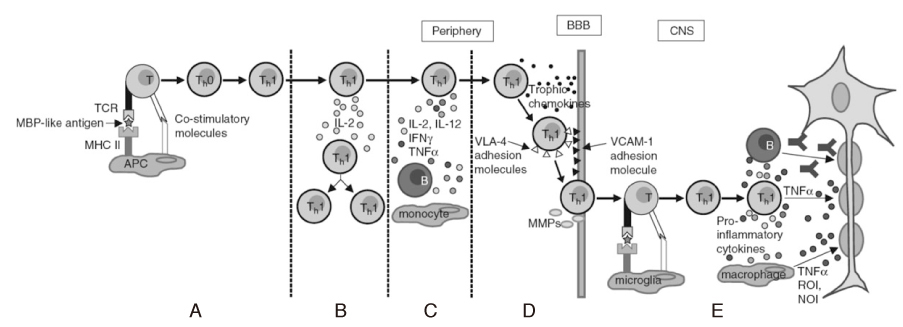
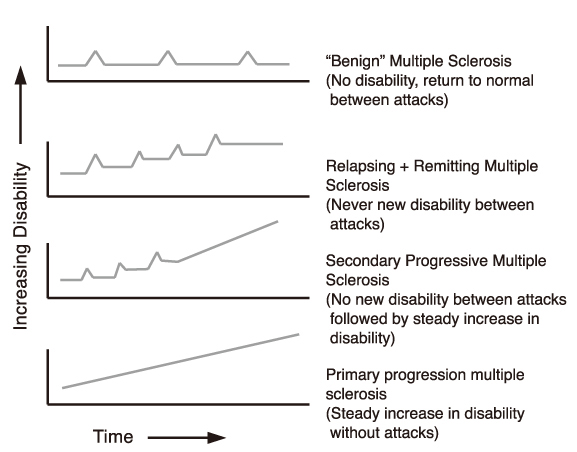
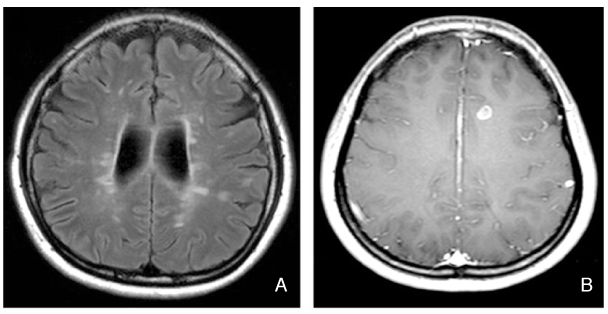
- Multiple sclerosis (MS) is an inflammatory autoimmune disorder of the central nervous system (CNS) and one of the most common disabling neurological diseases of young adults. Although the exact mechanisms involved in MS pathogenesis remain unclear, MS is believed to be caused by interactions between as yet unidentified environmental factors and susceptibility genes. Symptoms commonly occurred in MS include visual disturbance; weakness; spasticity; sensory disturbances; ataxia; bladder, bowel, and sexual dysfunction; fatigue; affective symptoms; and cognitive impairment. Most patients initially undergo a relapsing-remitting course, however, without treatment, the majority of them make a transition to the secondary progressive form. The clinical diagnosis is based on demonstrating neurological lesions, predominantly in the white matter, that are disseminated over space with the lapse of time. The key to the successful MS management is to prevent disability. Although there is no effective cure for MS, therapies are available that mitigate the course of the disease, treat relapses and improve symptoms, all of which place a significant impact on patients' quality of life. Recent clinical trials suggest that early identification and treatment are critical to optimize the treatment benefit. Currently six agents have been specifically approved for mitigating the course of MS. These include three formulations of interferon beta, glatiramer acetate, mitoxantrone, and natalizumab. Recent advances in understanding of immune pathogenesis lead us to new therapeutic approaches focused on precise target mechanisms. Many ongoing clinical trials will provide better treatment protocols in near future.
Keyword
MeSH Terms
-
Antibodies, Monoclonal, Humanized
Central Nervous System
Humans
Hypogonadism
Interferon-beta
Mitochondrial Diseases
Mitoxantrone
Multiple Sclerosis
Natalizumab
Ophthalmoplegia
Peptides
Quality of Life
Recurrence
Urinary Bladder
Young Adult
Antibodies, Monoclonal, Humanized
Hypogonadism
Interferon-beta
Mitochondrial Diseases
Mitoxantrone
Ophthalmoplegia
Peptides
Figure
Cited by 2 articles
-
Multiple sclerosis
Kwang-Kuk Kim
J Korean Med Assoc. 2013;56(8):702-708. doi: 10.5124/jkma.2013.56.8.702.A Case of Neuromyelitis Optica Misdiagnosed as Cervicogenic Headache
Soo Il Choi, Yeon Ju Lee, Do Wan Kim, Jong Yeun Yang
Korean J Pain. 2014;27(1):77-80. doi: 10.3344/kjp.2014.27.1.77.
Reference
-
1. Ropper AH, Brown RH. Adams and Victor's principles of neurology. 2005. 8th ed. New York: McGraw-Hill;771–796.2. Compston A. Genetic epidemiology of multiple sclerosis. J Neurol Neurosurg Psychiatry. 1997. 62:553–561.
Article3. Kurtzke JF. Mutliple sclerosis in time and space-geographic clues to cause. J Neurovirol. 2000. 6:134–140.4. Raghuwanshi A, Joshi SS, Christakos S. Vitamin D and multiple sclerosis. J Cell Biochem. 2008. 105:338–343.
Article5. Gale CR, Martyn CN. Migrant studies in multiple sclerosis. Prog Neurobiol. 1995. 47:425–448.
Article6. Sibley WA, Bamford CR, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985. 1:1313–1315.
Article7. Willer CJ, Dyment DA, Risch NJ, Sadovnick AD, Ebers GC. Canadian Collaborative Study Group. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc Natl Acad Sci USA. 2000. 100:12877–12882.8. Olerup O, Hillert J. HLA class II-associated genetic susceptibility in multiple sclerosis: a critical evaluation. Tissue Antigens. 1991. 38:1–15.
Article9. Svejgaard A. The immunogenetics of multiple sclerosis. Immunogenetics. 2008. 60:275–286.
Article10. Chofflon M. Mechanisms of action for treatments in multiple sclerosis; Does a heterogeneous disease demand a multi-targeted therapeutic approach? BioDrugs. 2005. 19:299–308.
Article11. Bar-Or A. Immunology of multiple sclerosis. Neurol Clin. 2005. 23:149–175.
Article12. Archelos JJ, Storch MK, Hartung HP. The role of B cells and autoantibodies in multiple sclerosis. Ann Neurol. 2000. 47:694–706.
Article13. Link H, Muller R. Immunoglobulins in multiple sclerosis and infections of the nervous system. Arch Neurol. 1971. 25:326–344.
Article14. Johnson KP, Nelson BJ. Multiple sclerosis: diagnostic usefulness of cerebrospinal fluid. Ann Neurol. 1977. 2:425–431.
Article15. Raine CS, Cannella B, Hause SL, Genain CP. Demyelination in primate autoimmune encephalopmyelitis and acute multiple sclerosis lesion: a case for antigen-speficit antibody mediation. Ann Neurol. 1999. 46:144–160.
Article16. Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesion: implications for the pathogenesis of demyelination. Ann Neurol. 2000. 47:707–717.
Article17. Buntinx M, Moreels M, Vandenabeele F, Lambrichts I, Raus J, Steels P, Stinissen P, Amellot M. Cytokine-induced cell death in human oligodendroglial cell lines: I. Synergistic effects of IFN-gamma and TNF-alpha on apoptosis. J Neurosci Res. 2004. 76:834–845.
Article18. Trapp BD, Peterson J, Ransohoff RM, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998. 29:278–285.
Article19. Hauser SL, Goodin DS. Braunwald E, Fauci AS, Kasper DL, Hauser SL, Longo DL, Jameson JL, Isselbacher K, editors. Multiple sclerosis and other demyelinating diseases. Harrison's Online. 2006. New York: McGraw-Hill;Available from: URL: http://www.accessmedicine.com.20. Rizzo JF III, Lessell S. Risk of developing multiple sclerosis after uncomplicated optic neuritis: A long-term prospective study. Neurology. 1988. 38:185–190.
Article21. Optic neuritis study group. The five-year risk of MS after optic neuritis. Experience of the optic neuritis treatment trial. Neurology. 1997. 49:1404–1413.22. Lucchinetti CF, Kiers L, O'Duffy A, Gomez MR, Cross S, Leavitt JA, O'Brien P, Rodriguez M. Risk factors for developing multiple sclerosis after childhood optic neuritis. Neurology. 1997. 49:1413–1418.
Article23. Beck RW, Trobe JD, Moke PS, Gal RL, Xing D, Bhatti MT, Brodsky MC, Buckley EG, Chrousos GA, Corbett J, Eggenberger E, Goodwin JA, Katz B, Kaufman DI, Keltner JL, Kupersmith MJ, Miller NR, Nazarian S, Orengo-Nania S, Savino PJ, Shults WT, Smith CH, Wall M. Optic neuritis study group. High-and low-risk profiles for the development of multiple sclerosis within 10 years after optic neuritis: experience of the optic neuritis treatment trial. Arch Ophthalmol. 2003. 121:944–949.
Article24. Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials on New Agents in Multiple Sclerosis. Neurology. 1996. 46:907–911.
Article25. Polman CH, Reingold SC, Edan G, Filippi M, Hartung HP, Kappos L, Lublin FD, Metz LM, McFarland HF, O'Connor PW, Wandberg-Wollheim M, Thompson AJ, Weinchenker BG, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann Neurol. 2005. 58:840–846.
Article26. Katzman GL. Osborn AG, Blaser SI, Salzman KL, Katzman GL, Provenzale J, Castillo M, Hedlund GL, Illner A, Harnsberger HR, Cooper JA, Jones BV, Hamilton BE, editors. Multiple sclerosis. Diagnostic imaging. Brain. 2004. Salt Lake City: Amirsys;I-8.74–I-8.77.27. Jiang H, Milo R, Swoveland P, Johnson KP, Panitch H, Dhib-Jalbut S. Interferon beta-1b reduces interferon gamma-induced antigen-presenting capacity of human glial and B cells. J Neuroimmunol. 1995. 61:17–25.28. Genc K, Dona DL, Reder AT. Increased CD80 (+) B cells in active multiple sclerosis and reversal by interferon beta-1b therapy. J Clin Invest. 1997. 99:2664–2671.29. Teleshova N, Bao W, Kivisakk P, Ozenci V, Mustafa M, Link H. Elevated CD40 ligand expressing blood T-cell levels in multiple sclerosis are reversed by interferon-beta treatment. Scand J Immunol. 2000. 51:312–320.
Article30. Sharief MK, Semra YK, Seidi OA, Zoukos Y. Interferon-beta therapy downregulates the anti-apoptosis protein FLIP in T cells from patients with multiple sclerosis. J Neuroimmunol. 2001. 120:199–207.
Article31. Calabresi PA, Pelfrey CM, Tranquill LR, Maloni H, McFarland HF. VLA-4 expression on peripheral blood lymphocytes is downregulated after treatment of multiple sclerosis with interferon beta. Neurology. 1997. 49:1111–1116.
Article32. Calabresi PA, Tranquill LR, Dambrosia JM, Stone LA, Maloni H, Bash CN, Frank JA, McFarland HF. Increases in soluble VCAM-1 correlate with a decrease in MRI lesions in multiple sclerosis treated with interferon beta-1b. Ann Neurol. 1997. 41:669–674.
Article33. Trojano M, Avolio C, Liuzzi GM, Ruggieri M, Defazio G, Liguori M, Santacroce MP, Paolicelli D, Giuliani F, Riccio P, Livrea P. Changes of serum sICAM-1 and MMP-9 induced by rIFNbeta-1b treatment in relapsing-remitting MS. Neurology. 1999. 53:1402–1408.
Article34. Calabresi PA, Stone LA, Bash CN, Frank JA, McFarland HF. Interferon beta results in immediate reduction of contrast-enhanced MRI lesions in multiple sclerosis patients followed by weekly MRI. Neurology. 1997. 48:1446–1448.
Article35. Petersen B, Bendtzen K, Koch-Henriksen N, Ravnborg M, Ross C, Sorensen PS. Danish multiple sclerosis group. Persistence of neutralizeing antibodies after discontinuation of IFN beta therapy in patients with relapsing-remitting multiple sclerosis. Mult Scler. 2006. 12:247–252.
Article36. Jacobs LD, Beck RW, Simon JH, Kinkel RP, Brownscheidle CM, Murray TJ, Simonian NA, Slasor PJ, Sandrock AW. CHAMPS Study Group. Intramuscular interferon beta-1a therapy initiated during a first demyelinating event in multiple sclerosis. N Engl J Med. 2000. 343:898–904.
Article37. Comi G, Filippi M, Barkhof F, Durelli L, Edan G, Fernández O, Hartung H, Seeldrayers P, Sørensen PS, Rovaris M, Martinelli V, Hommes OR. Early Treatment of Multiple Sclerosis Study Group. Effect of early interferon treatment on conversion to definite multiple sclerosis: a randomised study. Lancet. 2001. 357:1576–1582.
Article38. Kappos L, Polman CH, Freedman MS, Edan G, Hartung HP, Miller DH, Montalban X, Barkhof F, Bauer L, Jakobs P, Pohl C, Sandbrink R. Treatment with interferon beta-1b delays conversion to clinically definite and McDonald MS in patients with clinically isolated syndromes. Neurology. 2006. 67:1242–1249.
Article39. Arnon R. The development of Cop 1 (Copaxone), and innovative drug for the treatment of multiple sclerosis: personal reflextions. Immunol Lett. 1996. 50:1–15.
Article40. Teitelbaum D, Meshorer A, Hirshfeld T, Arnon R, Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971. 1:242–248.
Article41. Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001. 56:702–708.
Article42. Weber MS, Starck M, Wagenpfeil S, Meinl E, Hohlfeld R, Farina C. Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain. 2004. 127:1370–1378.
Article43. Kim HJ, Ifergan I, Antel JP, Seguin R, Duddy M, Lapierre Y, Jalili F, Bar-Or A. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol. 2004. 172:7144–7153.
Article44. Fox EJ. Mechanism of action of mitoxantrone. Neurology. 2004. 63:15–18.
Article45. Niino M, Bodner C, Simard ML, Alatab S, Gano D, Kim HJ, Trigueiro M, Racicot D, Guérette C, Antel JP, Fournier A, Grand'Maison F, Bar-Or A. Natalizumab effects on immune cell responses in multiple sclerosis. Ann Neurol. 2006. 59:748–754.
Article46. Miller DH, Khan OA, Sheremata WA, Blumhardt LD, Rice GP, Libonati MA, Willmer-Hulme AJ, Dalton CM, Miszkiel KA, O'Connor PW. International natalizumab multiple sclerosis trial group. A controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2003. 348:15–23.
Article47. Kleinschmidt-DeMasters BK, Tyler KL. Progressive multifocal leukoencephalopathy complicating treatment with natalizumab and interferon-b-1a for multiple sclerosis. N Engl J Med. 2005. 353:369–374.
Article48. Langer-Gould A, Atlas SW, Green AJ, Bollen AW, Pelletier D. Progressive multifocal leukoencephalopathy after natalizumab therapy for Crohn's disease. N Engl J Med. 2005. 353:362–368.
Article49. Buttmann M, Rieckmann P. Treating multiple sclerosis with monoclonal antibodies. Expert Rev Neurother. 2008. 8:433–455.
Article50. Cohen BA, Reickmann P. Emerging oral therapies for multiple sclerosis. Int J Clin Pract. 2007. 61:1922–1930.
Article51. Kesselring J, Beer S. Symptomatic therapy and neurorehabilitation in multiple sclerosis. Lancet Neurol. 2005. 4:643–652.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Reduction of Disease Activity in Patient with Relapsing-Remitting Multiple Sclerosis after Switching to Teriflunomide from Interferon Beta
- A Case of Multiple Sclerosis in Child Showing Homonymous Hemianopia
- Analgesia for Labor in a Parturient with Multiple Sclerosis: A case report
- A Case of Unilateral Renal Angiomyolipoma Associated with Tuberous Sclerosis
- Understanding MRI Features of Multiple Sclerosis: Based on the 2017 McDonald Criteria