Restor Dent Endod.
2017 Aug;42(3):176-187. 10.5395/rde.2017.42.3.176.
White mineral trioxide aggregate mixed with calcium chloride dihydrate: chemical analysis and biological properties
- Affiliations
-
- 1Universiti Sains Malaysia School of Dental Sciences, Kubang Kerian, Malaysia. hany_endodontist@hotmail.com
- 2Human Genome Centre, Universiti Sains Malaysia School of Medical Sciences, Kubang Kerian, Malaysia.
- 3International Islamic University Malaysia, Kulliyyah of Dentistry, Kuantan, Malaysia.
- KMID: 2386771
- DOI: http://doi.org/10.5395/rde.2017.42.3.176
Abstract
OBJECTIVES
This study aimed to evaluate the chemical and biological properties of fast-set white mineral trioxide aggregate (FS WMTA), which was WMTA combined with calcium chloride dihydrate (CaClâ‚‚·2Hâ‚‚O), compared to that of WMTA.
MATERIALS AND METHODS
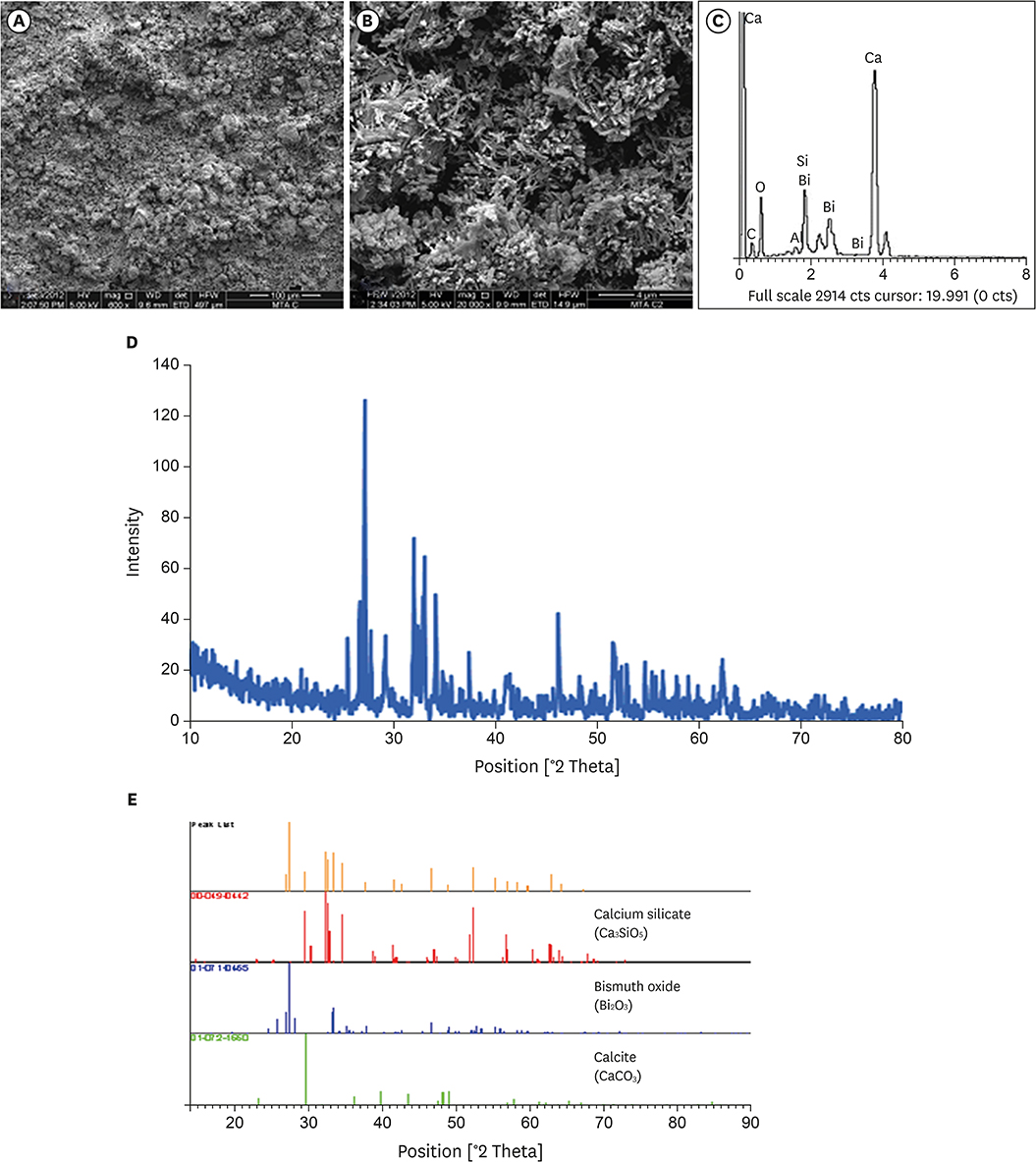
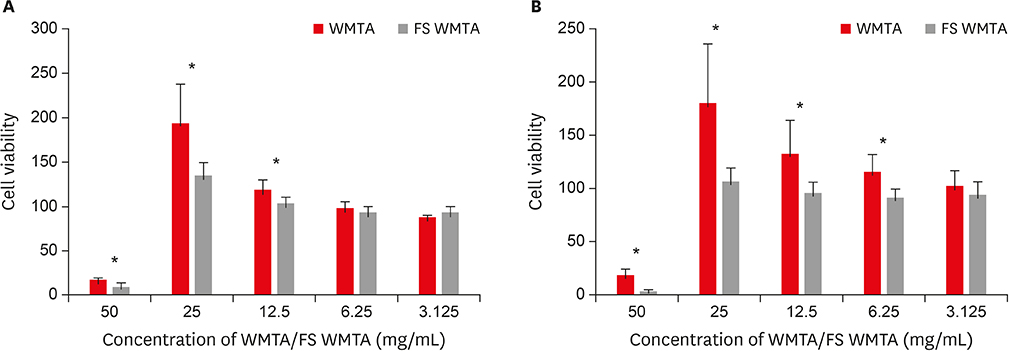
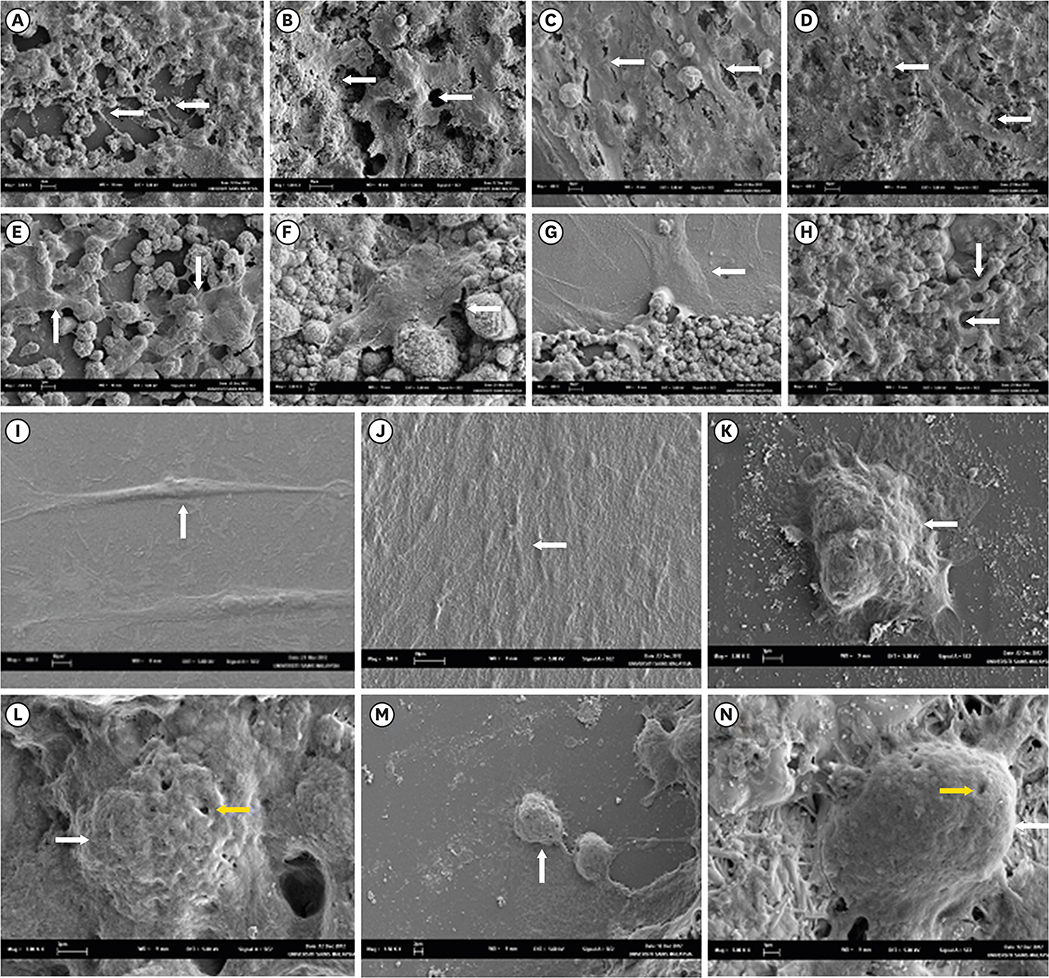
Surface morphology, elemental, and phase analysis were examined using scanning electron microscope (SEM), energy dispersive X-ray microanalysis (EDX), and X-ray diffraction (XRD), respectively. The cytotoxicity and cell attachment properties were evaluated on human periodontal ligament fibroblasts (HPLFs) using methyl-thiazol-diphenyltetrazolium (MTT) assay and under SEM after 24 and 72 hours, respectively.
RESULTS
Results showed that the addition of CaClâ‚‚·2Hâ‚‚O to WMTA affected the surface morphology and chemical composition. Although FS WMTA exhibited a non-cytotoxic profile, the cell viability values of this combination were lesser than WMTA, and the difference was significant in 7 out of 10 concentrations at the 2 time intervals (p < 0.05). HPLFs adhered over the surface of WMTA and at the interface, after 24 hours of incubation. After 72 hours, there were increased numbers of HPLFs with prominent cytoplasmic processes. Similar findings were observed with FS WMTA, but the cells were not as confluent as with WMTA.
CONCLUSIONS
The addition of CaClâ‚‚·2Hâ‚‚O to WMTA affected its chemical properties. The favorable biological profile of FS WMTA towards HPLFs may have a potential impact on its clinical application for repair of perforation defects.
Keyword
MeSH Terms
Figure
Reference
-
1. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993; 19:591–595.
Article2. Al-Rabeah E, Perinpanayagam H, MacFarland D. Human alveolar bone cells interact with ProRoot and tooth-colored MTA. J Endod. 2006; 32:872–875.
Article3. Nair PN, Duncan HF, Pitt Ford TR, Luder HU. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: a randomized controlled trial. Int Endod J. 2008; 41:128–150.
Article4. Torabinejad M, Parirokh M. Mineral trioxide aggregate: a comprehensive literature review--part II: leakage and biocompatibility investigations. J Endod. 2010; 36:190–202.
Article5. Hakki SS, Bozkurt SB, Ozcopur B, Purali N, Belli S. Periodontal ligament fibroblast response to root perforations restored with different materials: a laboratory study. Int Endod J. 2012; 45:240–248.
Article6. Wiltbank KB, Schwartz SA, Schindler WG. Effect of selected accelerants on the physical properties of mineral trioxide aggregate and Portland cement. J Endod. 2007; 33:1235–1238.
Article7. Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review--Part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010; 36:400–413.
Article8. Lee ES. A new mineral trioxide aggregate root-end filling technique. J Endod. 2000; 26:764–765.
Article9. Kogan P, He J, Glickman GN, Watanabe I. The effects of various additives on setting properties of MTA. J Endod. 2006; 32:569–572.
Article10. Huang TH, Shie MY, Kao CT, Ding SJ. The effect of setting accelerator on properties of mineral trioxide aggregate. J Endod. 2008; 34:590–593.
Article11. Lee BN, Hwang YC, Jang JH, Chang HS, Hwang IN, Yang SY, Park YJ, Son HH, Oh WM. Improvement of the properties of mineral trioxide aggregate by mixing with hydration accelerators. J Endod. 2011; 37:1433–1436.
Article12. Ahmed HMA, Saini R, Rahman IA, Saini D. Effect of bee products on the setting properties of mineral trioxide aggregate mixed with calcium chloride dihydrate. A preliminary study. J ApiProduct ApiMedical Sci. 2011; 3:123–127.
Article13. Ber BS, Hatton JF, Stewart GP. Chemical modification of proroot mta to improve handling characteristics and decrease setting time. J Endod. 2007; 33:1231–1234.
Article14. Lee BN, Kim HJ, Chang HS, Hwang IN, Oh WM, Kim JW, Koh JT, Min KS, Choi CH, Hwang YC. Effects of mineral trioxide aggregate mixed with hydration accelerators on osteoblastic differentiation. J Endod. 2014; 40:2019–2023.
Article15. Zapf AM, Chedella SC, Berzins DW. Effect of additives on mineral trioxide aggregate setting reaction product formation. J Endod. 2015; 41:88–91.
Article16. Prasad A, Pushpa S, Arunagiri D, Sawhny A, Misra A, Sujatha R. A comparative evaluation of the effect of various additives on selected physical properties of white mineral trioxide aggregate. J Conserv Dent. 2015; 18:237–241.
Article17. Kulan P, Karabiyik O, Kose GT, Kargul B. Biocompatibility of accelerated mineral trioxide aggregate on stem cells derived from human dental pulp. J Endod. 2016; 42:276–279.
Article18. Antunes Bortoluzzi E, Juárez Broon N, Antonio Hungaro Duarte M, de Oliveira Demarchi AC, Monteiro Bramante C. The use of a setting accelerator and its effect on pH and calcium ion release of mineral trioxide aggregate and white Portland cement. J Endod. 2006; 32:1194–1197.
Article19. Bortoluzzi EA, Broon NJ, Bramante CM, Felippe WT, Tanomaru Filho M, Esberard RM. The influence of calcium chloride on the setting time, solubility, disintegration, and pH of mineral trioxide aggregate and white Portland cement with a radiopacifier. J Endod. 2009; 35:550–554.
Article20. Bortoluzzi EA, Broon NJ, Bramante CM, Consolaro A, Garcia RB, de Moraes IG, Bernadineli N. Mineral trioxide aggregate with or without calcium chloride in pulpotomy. J Endod. 2008; 34:172–175.
Article21. Jafarnia B, Jiang J, He J, Wang YH, Safavi KE, Zhu Q. Evaluation of cytotoxicity of MTA employing various additives. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:739–744.
Article22. Kang JY, Lee BN, Son HJ, Koh JT, Kang SS, Son HH, Chang HS, Hwang IN, Hwang YC, Oh WM. Biocompatibility of mineral trioxide aggregate mixed with hydration accelerators. J Endod. 2013; 39:497–500.
Article23. Ahmed HMA, Luddin N, Kannan TP, Mokhtar KI, Ahmad A. Chemical analysis and biological properties of two different formulations of white Portland cements. Scanning. 2016; 38:303–316.
Article24. Ong RM, Luddin N, Ahmed HMA, Omar NS. Cytotoxicity of accelerated white MTA and Malaysian white Portland cement on stem cells from human exfoliated deciduous teeth (SHED): an in vitro study. Singapore Dent J. 2012; 33:19–23.
Article25. Camilleri J. Characterization and chemical activity of Portland cement and two experimental cements with potential for use in dentistry. Int Endod J. 2008; 41:791–799.
Article26. Hwang YC, Lee SH, Hwang IN, Kang IC, Kim MS, Kim SH, Son HH, Oh WM. Chemical composition, radiopacity, and biocompatibility of Portland cement with bismuth oxide. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e96–e102.
Article27. Ahmed HMA, Omar NS, Luddin N, Saini R, Saini D. Cytotoxicity evaluation of a new fast set highly viscous conventional glass ionomer cement with L929 fibroblast cell line. J Conserv Dent. 2011; 14:406–408.
Article28. Zhang W, Li Z, Peng B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int Endod J. 2010; 43:769–774.
Article29. Ahmed HMA, Luddin N, Kannan TP, Mokhtar KI, Ahmad A. Cell attachment properties of Portland cement-based endodontic materials: biological and methodological considerations. J Endod. 2014; 40:1517–1523.
Article30. Traetteberg A, Ramachandran VS, Grattan-Bellew PE. A study of the microstructure and hydration characteristics of tricalcium silicate in the presence of calcium chloride. Cement Concr Res. 1974; 4:203–221.
Article31. Wang X, Sun H, Chang J. Characterization of Ca3SiO5/CaCl2 composite cement for dental application. Dent Mater. 2008; 24:74–82.32. Oliveira MG, Xavier CB, Demarco FF, Pinheiro AL, Costa AT, Pozza DH. Comparative chemical study of MTA and Portland cements. Braz Dent J. 2007; 18:3–7.
Article33. Asgary S, Parirokh M, Eghbal MJ, Brink F. A comparative study of white mineral trioxide aggregate and white Portland cements using X-ray microanalysis. Aust Endod J. 2004; 30:89–92.
Article34. Dammaschke T, Gerth HU, Züchner H, Schäfer E. Chemical and physical surface and bulk material characterization of white ProRoot MTA and two Portland cements. Dent Mater. 2005; 21:731–738.
Article35. Camilleri J, Montesin FE, Brady K, Sweeney R, Curtis RV, Ford TR. The constitution of mineral trioxide aggregate. Dent Mater. 2005; 21:297–303.
Article36. Song JS, Mante FK, Romanow WJ, Kim S. Chemical analysis of powder and set forms of Portland cement, gray ProRoot MTA, white ProRoot MTA, and gray MTA-Angelus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006; 102:809–815.
Article37. Belío-Reyes IA, Bucio L, Cruz-Chavez E. Phase composition of ProRoot mineral trioxide aggregate by X-ray powder diffraction. J Endod. 2009; 35:875–878.
Article38. Al-Hezaimi K, Al-Shalan TA, Naghshbandi J, Simon JH, Rotstein I. MTA preparations from different origins may vary in their antimicrobial activity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009; 107:e85–e88.
Article39. Camilleri J, Montesin FE, Di Silvio L, Pitt Ford TR. The chemical constitution and biocompatibility of accelerated Portland cement for endodontic use. Int Endod J. 2005; 38:834–842.
Article40. Islam I, Chng HK, Yap AU. X-ray diffraction analysis of mineral trioxide aggregate and Portland cement. Int Endod J. 2006; 39:220–225.
Article41. Park JW, Hong SH, Kim JH, Lee SJ, Shin SJ. X-Ray diffraction analysis of white ProRoot MTA and Diadent BioAggregate. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:155–158.
Article42. Taylor HF. Cement chemistry. 2nd ed. London: Thomas Telford Ltd.;1997. p. 321–322.43. Al-Hiyasat AS, Al-Sa'Eed OR, Darmani H. Quality of cellular attachment to various root-end filling materials. J Appl Oral Sci. 2012; 20:82–88.
Article44. Keiser K, Johnson CC, Tipton DA. Cytotoxicity of mineral trioxide aggregate using human periodontal ligament fibroblasts. J Endod. 2000; 26:288–291.
Article45. Watts JD, Holt DM, Beeson TJ, Kirkpatrick TC, Rutledge RE. Effects of pH and mixing agents on the temporal setting of tooth-colored and gray mineral trioxide aggregate. J Endod. 2007; 33:970–973.
Article46. Boutsioukis C, Noula G, Lambrianidis T. Ex vivo study of the efficiency of two techniques for the removal of mineral trioxide aggregate used as a root canal filling material. J Endod. 2008; 34:1239–1242.
Article47. Belobrov I, Parashos P. Treatment of tooth discoloration after the use of white mineral trioxide aggregate. J Endod. 2011; 37:1017–1020.
Article48. Thomson TS, Berry JE, Somerman MJ, Kirkwood KL. Cementoblasts maintain expression of osteocalcin in the presence of mineral trioxide aggregate. J Endod. 2003; 29:407–412.
Article49. Zhu Q, Haglund R, Safavi KE, Spangberg LS. Adhesion of human osteoblasts on root-end filling materials. J Endod. 2000; 26:404–406.
Article50. Ma J, Shen Y, Stojicic S, Haapasalo M. Biocompatibility of two novel root repair materials. J Endod. 2011; 37:793–798.
Article51. Asgary S, Moosavi SH, Yadegari Z, Shahriari S. Cytotoxic effect of MTA and CEM cement in human gingival fibroblast cells. Scanning electronic microscope evaluation. N Y State Dent J. 2012; 78:51–54.52. Trichaiyapon V, Torrungruang K, Panitvisai P. Cytotoxicity of flowable resin composite on cultured human periodontal ligament cells compared with mineral trioxide aggregate. J Investig Clin Dent. 2012; 3:215–220.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemical characteristics of mineral trioxide aggregate and its hydration reaction
- Physical and chemical properties of experimental mixture of mineral trioxide aggregate and glass ionomer cement
- Evaluation of cytotoxicity and inflammatory tissue response of mineral trioxide aggregates containing dicalcium phosphate dihydrate
- Perspective of endodontic sealers based on calcium silicate
- Discoloration and radiopacity of white mineral trioxide aggregate with various radiopacifiers